Page 789 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 789
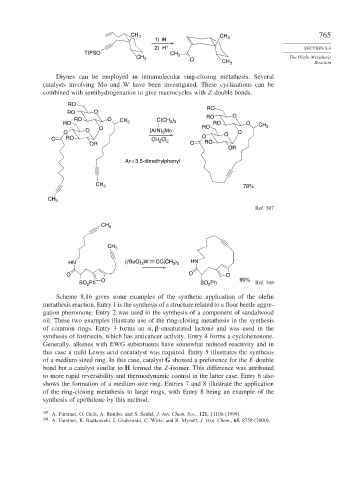
CH 3 CH 3 765
1) H
2) H + SECTION 8.4
TIPSO CH 3
CH 3 O CH 3 The Olefin Metathesis
Reaction
Diynes can be employed in intramolecular ring-closing metathesis. Several
catalysts involving Mo and W have been investigated. These cyclizations can be
combined with semihydrogenation to give macrocycles with Z-double bonds.
RO
RO
RO O
RO O
RO O CH C(CH )
RO 3 3 3 RO O CH
O RO 3
O (ArN) Mo
O 3 O O
O RO CH Cl 2 O
2
OR O RO
OR
Ar = 3,5-dimethylphenyl
CH 3 78%
CH 3
Ref. 307
CH 3
CH 3
HN (t BuO) W CC(CH ) HN
3 3
3
O O O
O 90%
SO Ph SO Ph Ref. 308
2
2
Scheme 8.16 gives some examples of the synthetic application of the olefin
metathesis reaction. Entry 1 is the synthesis of a structure related to a flour beetle aggre-
gation pheromone. Entry 2 was used in the synthesis of a component of sandalwood
oil. These two examples illustrate use of the ring-closing metathesis in the synthesis
of common rings. Entry 3 forms an -unsaturated lactone and was used in the
synthesis of fostriecin, which has anticancer activity. Entry 4 forms a cyclohexenone.
Generally, alkenes with EWG substituents have somewhat reduced reactivity and in
this case a mild Lewis acid cocatalyst was required. Entry 5 illustrates the synthesis
of a medium-sized ring. In this case, catalyst G showed a preference for the E-double
bond but a catalyst similar to H formed the Z-isomer. This difference was attributed
to more rapid reversibility and thermodynamic control in the latter case. Entry 6 also
shows the formation of a medium-size ring. Entries 7 and 8 illustrate the application
of the ring-closing metathesis to large rings, with Entry 8 being an example of the
synthesis of epothilone by this method.
307 A. Furstner, O. Guth, A. Rumbo, and S. Seidel, J. Am. Chem. Soc., 121, 11108 (1999).
308
A. Furstner, K. Radkowski, J. Grabowski, C. Wirtz, and R. Mynott, J. Org. Chem., 65, 8758 (2000).