Page 787 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 787
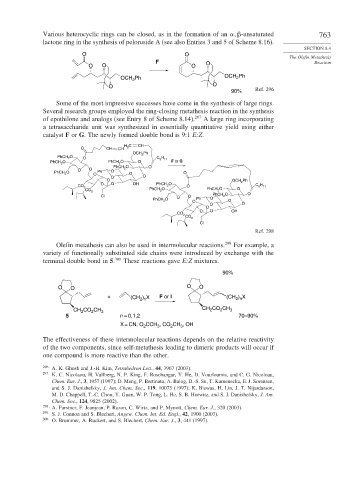
Various heterocyclic rings can be closed, as in the formation of an -unsaturated 763
lactone ring in the synthesis of peloruside A (see also Entries 3 and 5 of Scheme 8.16).
SECTION 8.4
O O
The Olefin Metathesis
F Reaction
O O O O
OCH Ph OCH Ph
2
2
O
O
90% Ref. 296
Some of the most impressive successes have come in the synthesis of large rings.
Several research groups employed the ring-closing metathesis reaction in the synthesis
of epothilone and analogs (see Entry 8 of Scheme 8.14). 297 A large ring incorporating
a tetrasaccharide unit was synthesized in essentially quantitative yield using either
catalyst F or G. The newly formed double bond is 9:1 E:Z.
H C CH
O CH CH 2
Ph
OCH 2
O
PhCH 2 O C H
PhCH O PhCH O O 5 11 F or G
2
2
PhCH O O
O O O 2
PhCH O Ph O
2 O O
O O
O OCH 2 Ph
CO 2 O O OH PhCH O O C H
2
5 11
PhCH 2 O PhCH O O
CO 2 2
O O
Cl O O
PhCH 2
PhCH O Ph O
2 O O
O O
O
O O OH
CO
2
CO 2
Cl
Ref. 298
Olefin metathesis can also be used in intermolecular reactions. 299 For example, a
variety of functionally substituted side chains were introduced by exchange with the
terminal double bond in 5. 300 These reactions gave E:Z mixtures.
90%
O O O O
+ (CH ) X F or I (CH ) X
2 n
2 n
CH CO CH 3 CH 2 CO CH 3
2
2
2
5 n = 0,1,2 70–90%
X = CN, O CCH , CO CH , OH
2
3
3
2
The effectiveness of these intermolecular reactions depends on the relative reactivity
of the two components, since self-metathesis leading to dimeric products will occur if
one compound is more reactive than the other.
296
A. K. Ghosh and J.-H. Kim, Tetrahedron Lett., 44, 3967 (2003).
297 K. C. Nicolaou, H. Vallberg, N. P. King, F. Roschangar, Y. He, D. Vourloumis, and C. G. Nicoloau,
Chem. Eur. J., 3, 1957 (1997); D. Meng, P. Bertinato, A. Balog, D.-S. Su, T. Kamenecka, E. J. Sorensen,
and S. J. Danishefsky, J. Am. Chem. Soc., 119, 10073 (1997); K. Biswas, H. Lin, J. T. Nijardarson,
M. D. Chappell, T.-C. Chou, Y. Guan, W. P. Tong, L. He, S. B. Horwitz, and S. J. Danishefsky, J. Am.
Chem. Soc., 124, 9825 (2002).
298
A. Furstner, F. Jeanjean, P. Razon, C. Wirtz, and P. Mynott, Chem. Eur. J., 320 (2003).
299 S. J. Connon and S. Blechert, Angew. Chem. Int. Ed. Engl., 42, 1900 (2003).
300
O. Brummer, A. Ruckert, and S. Blechert, Chem. Eur. J., 3, 441 (1997).