Page 799 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 799
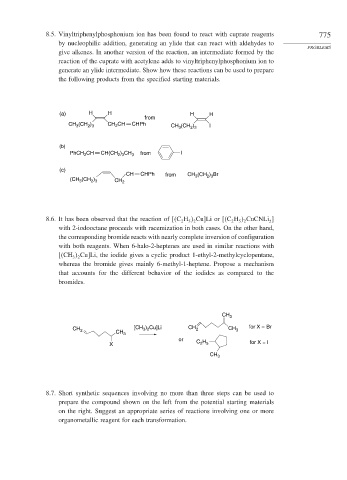
8.5. Vinyltriphenylphosphonium ion has been found to react with cuprate reagents 775
by nucleophilic addition, generating an ylide that can react with aldehydes to
PROBLEMS
give alkenes. In another version of the reaction, an intermediate formed by the
reaction of the cuprate with acetylene adds to vinyltriphenylphosphonium ion to
generate an ylide intermediate. Show how these reactions can be used to prepare
the following products from the specified starting materials.
(a) H H H H
from
(CH ) CH CHPh
CH 3 2 3 CH 2 CH (CH ) I
2 3
3
(b)
PhCH CH CH(CH ) CH 3 from I
2
2 3
(c)
CH CHPh from CH 3 (CH 2 ) 3 Br
(CH (CH ) CH 2
2 3
3
8.6. It has been observed that the reaction of C H Cu
Li or C H CuCNLi ]
2
5 2
2
5 2
2
with 2-iodooctane proceeds with racemization in both cases. On the other hand,
the corresponding bromide reacts with nearly complete inversion of configuration
with both reagents. When 6-halo-2-heptenes are used in similar reactions with
CH Cu
Li, the iodide gives a cyclic product 1-ethyl-2-methylcyclopentane,
3 2
whereas the bromide gives mainly 6-methyl-1-heptene. Propose a mechanism
that accounts for the different behavior of the iodides as compared to the
bromides.
CH 3
CH 2 CH 3 [CH ) Cu]Li CH 2 CH 3 for X = Br
3 2
or C H
X 2 5 for X = I
CH 3
8.7. Short synthetic sequences involving no more than three steps can be used to
prepare the compound shown on the left from the potential starting materials
on the right. Suggest an appropriate series of reactions involving one or more
organometallic reagent for each transformation.