Page 802 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 802
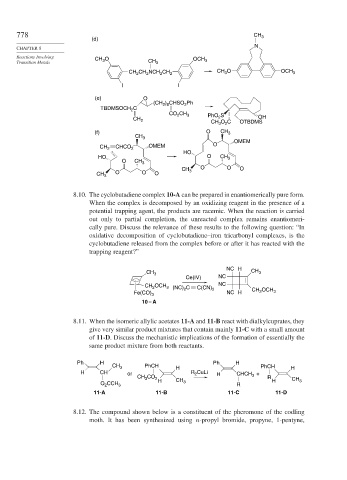
778 CH 3
(d)
N
CHAPTER 8
Reactions Involving CH 3 O OCH
Transition Metals CH 3 3
CH CH NCH CH 2 CH O OCH 3
3
2
2
2
I I
(e) O
(CH ) CHSO Ph
2
2 8
TBDMSOCH C
2
CO CH 3 PhO S
2
CH 2 2 OH
CH O C OTBDMS
3
2
(f) O CH 3
CH 3
OMEM
CH 2 CHCO 2 OMEM O
HO
HO O CH 3
O CH 3
O O O
CH 3
CH 3 O O O
8.10. The cyclobutadiene complex 10-A can be prepared in enantiomerically pure form.
When the complex is decomposed by an oxidizing reagent in the presence of a
potential trapping agent, the products are racemic. When the reaction is carried
out only to partial completion, the unreacted complex remains enantiomeri-
cally pure. Discuss the relevance of these results to the following question: “In
oxidative decomposition of cyclobutadiene–iron tricarbonyl complexes, is the
cyclobutadiene released from the complex before or after it has reacted with the
trapping reagent?”
NC H
CH 3 CH 3
Ce(IV) NC
CH OCH 3 (NC) C C(CN) NC
2
2
Fe(CO) 3 2 2 NC H CH OCH 3
10 – A
8.11. When the isomeric allylic acetates 11-A and 11-B react with dialkylcuprates, they
give very similar product mixtures that contain mainly 11-C with a small amount
of 11-D. Discuss the mechanistic implications of the formation of essentially the
same product mixture from both reactants.
Ph H Ph H
CH 3 PhCH H PhCH H
H CH or R CuLi H CHCH 3 +
2
CH 3 CO 2 R CH
H CH 3 H 3
O CCH 3 R
2
11-A 11-B 11-C 11-D
8.12. The compound shown below is a constituent of the pheromone of the codling
moth. It has been synthesized using n-propyl bromide, propyne, 1-pentyne,