Page 806 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 806
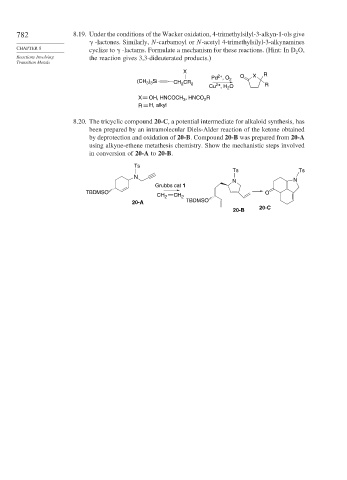
782 8.19. Under the conditions of the Wacker oxidation, 4-trimethylsilyl-3-alkyn-1-ols give
-lactones. Similarly, N-carbamoyl or N-acetyl 4-trimethylsilyl-3-alkynamines
CHAPTER 8 cyclize to -lactams. Formulate a mechanism for these reactions. (Hint: In D O,
2
Reactions Involving the reaction gives 3,3-dideuterated products.)
Transition Metals
X R
2+
Pd , O O X
(CH ) Si CH CR 2
3 3
2
2
2+
Cu , H O R
2
X OH, HNCOCH , HNCO R
3
2
R H, alkyl
8.20. The tricyclic compound 20-C, a potential intermediate for alkaloid synthesis, has
been prepared by an intramolecular Diels-Alder reaction of the ketone obtained
by deprotection and oxidation of 20-B. Compound 20-B was prepared from 20-A
using alkyne-ethene metathesis chemistry. Show the mechanistic steps involved
in conversion of 20-A to 20-B.
Ts
Ts Ts
N
N N
Grubbs cat 1
TBDMSO O
CH 2 CH 2
20-A TBDMSO
20-C
20-B