Page 803 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 803
ethylene oxide, and CO as the source of the carbon atoms. Devise a route for 779
2
such a synthesis. Hint: Extensive use of organocopper reagents is the basis for
PROBLEMS
the synthesis.
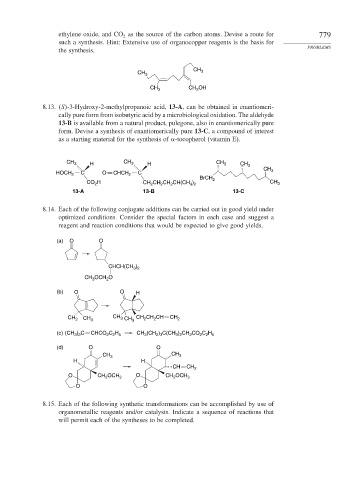
CH
CH 3 3
CH 3 CH OH
2
8.13. S -3-Hydroxy-2-methylpropanoic acid, 13-A, can be obtained in enantiomeri-
cally pure form from isobutyric acid by a microbiological oxidation. The aldehyde
13-B is available from a natural product, pulegone, also in enantiomerically pure
form. Devise a synthesis of enantiomerically pure 13-C, a compound of interest
as a starting material for the synthesis of -tocopherol (vitamin E).
CH CH
CH 3 H 3 H 3 CH 3
CH
HOCH 2 C O CHCH 2 C 3
BrCH 2
CO H CH CH CH CH(CH ) CH 3
3 2
2
2
2
2
13-A 13-B 13-C
8.14. Each of the following conjugate additions can be carried out in good yield under
optimized conditions. Consider the special factors in each case and suggest a
reagent and reaction conditions that would be expected to give good yields.
(a) O O
)
CHCH(CH 3 2
CH OCH O
3
2
(b) O O H
CH 3 CH 3 CH 3 CH 3 CH CH CH CH 2
2
2
(c) (CH ) C CHCO C H CH (CH ) C(CH ) CH CO C H
2
3 2
2 3
3 2
2 2 5
2 2 5
3
(d) O O
CH
CH 3 3
H H
CH CH 2
O CH OCH 3 O CH OCH 3
2
2
O O
8.15. Each of the following synthetic transformations can be accomplished by use of
organometallic reagents and/or catalysts. Indicate a sequence of reactions that
will permit each of the syntheses to be completed.