Page 861 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 861
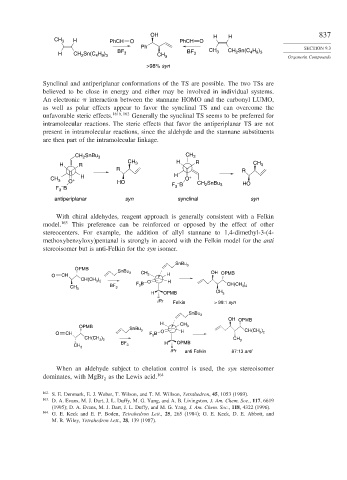
OH H H 837
CH 3 H PhCH O PhCH O
Ph SECTION 9.3
2
4 9 3
H CH Sn(C H ) BF 3 CH 3 BF 3 CH 3 CH Sn(C H ) Organotin Compounds
2
4 9 3
>98% syn
Synclinal and antiperiplanar conformations of the TS are possible. The two TSs are
believed to be close in energy and either may be involved in individual systems.
An electronic interaction between the stannane HOMO and the carbonyl LUMO,
as well as polar effects appear to favor the synclinal TS and can overcome the
unfavorable steric effects. 161b
162 Generally the synclinal TS seems to be preferred for
intramolecular reactions. The steric effects that favor the antiperiplanar TS are not
present in intramolecular reactions, since the aldehyde and the stannane substituents
are then part of the intramolecular linkage.
CH SnBu 3 CH 3
2
CH H R
H R 3 CH 3
R R
CH 3 O + H HO H – O + CH SnBu HO
–
F B F 3 B 2 3
3
antiperiplanar syn synclinal syn
With chiral aldehydes, reagent approach is generally consistent with a Felkin
model. 163 This preference can be reinforced or opposed by the effect of other
stereocenters. For example, the addition of allyl stannane to 1,4-dimethyl-3-(4-
methoxybenzyloxy)pentanal is strongly in accord with the Felkin model for the anti
stereoisomer but is anti-Felkin for the syn isomer.
SnBu
3
OPMB
SnBu 3 CH OH OPMB
O CH 3 H
)
CH(CH 3 2 O H
F B )
BF 3 CH(CH 3 2
CH 3 3
H OPMB CH 3
iPr Felkin > 99:1 syn
SnBu 3
OH OPMB
H CH
OPMB 3
SnBu 3 H )
O CH F B O CH(CH 3 2
CH(CH ) 3
3 2 CH 3
BF H OPMB
CH 3
3
iPr anti Felkin 87:13 anti
When an aldehyde subject to chelation control is used, the syn stereoisomer
dominates, with MgBr as the Lewis acid. 164
2
162 S. E. Denmark, E. J. Weber, T. Wilson, and T. M. Willson, Tetrahedron, 45, 1053 (1989).
163 D. A. Evans, M. J. Dart, J. L. Duffy, M. G. Yang, and A. B. Livingston, J. Am. Chem. Soc., 117, 6619
(1995); D. A. Evans, M. J. Dart, J. L. Duffy, and M. G. Yang, J. Am. Chem. Soc., 118, 4322 (1996).
164
G. E. Keck and E. P. Boden, Tetrahedron Lett., 25, 265 (1984); G. E. Keck, D. E. Abbott, and
M. R. Wiley, Tetrahedron Lett., 28, 139 (1987).