Page 864 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 864
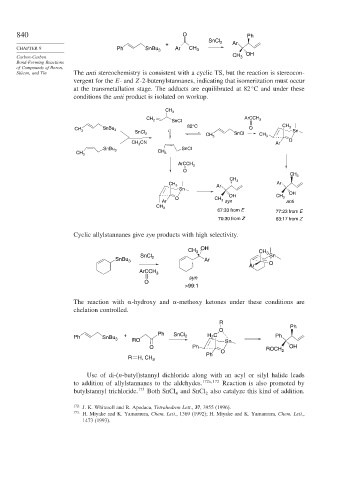
840 O Ph
SnCl 2
+ Ar
CHAPTER 9 Ph SnBu 3 Ar CH 3
Carbon-Carbon CH 3 OH
Bond-Forming Reactions
of Compounds of Boron,
Silicon, and Tin The anti stereochemistry is consistent with a cyclic TS, but the reaction is stereocon-
vergent for the E- and Z-2-butenylstannanes, indicating that isomerization must occur
at the transmetallation stage. The adducts are equilibrated at 82 C and under these
conditions the anti product is isolated on workup.
CH 3
CH 2 SnCl ArCCH 3
SnBu 82°C O CH 3
CH 3 3 Sn
SnCl SnCl
2 CH
CH 3 3
O
CH 3 CN Ar
SnBu SnCl
3
CH 3
CH 3
ArCCH 3
O
CH 3
CH 3
CH 3 Ar Ar
Sn
OH
OH CH
O CH 3
Ar 3 syn anti
CH 3
67:33 from E 77:23 from E
70:30 from Z 83:17 from Z
Cyclic allylstannanes give syn products with high selectivity.
CH 3 OH CH
SnCl 3 Sn
SnBu 3 2 Ar
Ar O
ArCCH 3
syn
O
>99:1
The reaction with -hydroxy and -methoxy ketones under these conditions are
chelation controlled.
R
Ph
O
Ph SnBu 3 + RO Ph SnCl 2 H 2 C Sn Ph
O Ph ROCH OH
O 2
Ph
R H, CH 3
Use of di-(n-butyl)stannyl dichloride along with an acyl or silyl halide leads
to addition of allylstannanes to the aldehydes. 172a
172 Reaction is also promoted by
butylstannyl trichloride. 173 Both SnCl and SnCl also catalyze this kind of addition.
4
2
172 J. K. Whitesell and R. Apodaca, Tetrahedron Lett., 37, 3955 (1996).
173
H. Miyake and K. Yamamura, Chem. Lett., 1369 (1992); H. Miyake and K. Yamamura, Chem. Lett.,
1473 (1993).