Page 868 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 868
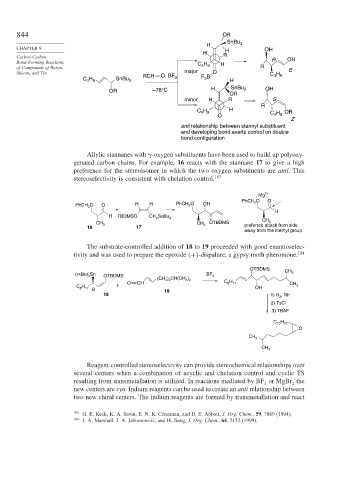
844 OR
H SnBu 3
CHAPTER 9 OH
H H
Carbon-Carbon R OR
Bond-Forming Reactions H R
C 4 9 H
of Compounds of Boron, R E
Silicon, and Tin O, BF major O C 4 9
H
3
C H SnBu 3 RCH 3 F B H
4 9
OR –78°C H SnBu 3 OH
OR
minor H R S
R
C H H H OR
4 9
O C 4 9
Z
anti relationship between stannyl substituent
and developing bond exerts control on double
bond configuration
Allylic stannanes with -oxygen substituents have been used to build up polyoxy-
genated carbon chains. For example, 16 reacts with the stannane 17 to give a high
preference for the stereoisomer in which the two oxygen substituents are anti. This
stereoselectivity is consistent with chelation control. 183
Mg 2+
PhCH O O
PhCH O O H H PhCH 2 O OH 2
2
+
H
H TBDMSO CH SnBu 3
2
CH CH OTBDMS CH 3
16 3 17 3 preferred attack from side
away from the methyl group
The substrate-controlled addition of 18 to 19 proceeded with good enantioselec-
tivity and was used to prepare the epoxide + -dispalure, a gypsy moth pheromone. 184
OTBDMS CH
(n-Bu) Sn OTBDMS BF 3 3
3
(CH ) CH(CH )
2 2
3 2
O CH C H 17 CH
8
H + 3
C 8 17 OH
S 19
18 1) H , Rh
2
2) TsCl
3) TBAF
C H 21
10
O
CH 3
CH 3
Reagent-controlled stereoselectivity can provide stereochemical relationships over
several centers when a combination of acyclic and chelation control and cyclic TS
resulting from transmetallation is utilized. In reactions mediated by BF or MgBr the
2
3
new centers are syn. Indium reagents can be used to create an anti relationship between
two new chiral centers. The indium reagents are formed by transmetallation and react
183 G. E. Keck, K. A. Savin, E. N. K. Cressman, and D. E. Abbott, J. Org. Chem., 59, 7889 (1994).
184
J. A. Marshall, J. A. Jablonowski, and H. Jiang, J. Org. Chem., 64, 2152 (1999).