Page 873 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 873
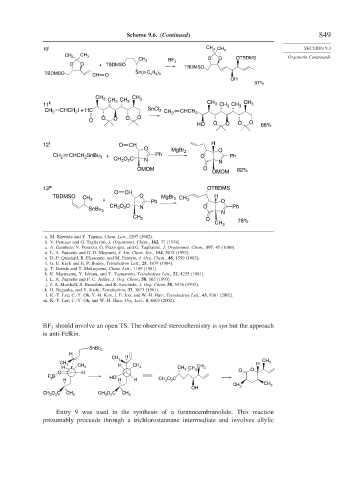
Scheme 9.6. (Continued) 849
10 j CH 3 CH 3 SECTION 9.3
CH CH
3 3 O O OTBDMS Organotin Compounds
CH 3 BF
O O + TBDMSO 3
TBDMSO
TBDMSO Sn(n-C H )
CH O 4 9 3
OH
97%
CH 3 CH CH 3 CH 3
11 k 3 CH 3 CH 3 CH 3 CH 3
CH 2 CHCH I + HC SnCl 2 CH 2 CHCH 2
2
O O O O
O O
HO O O O 68%
12 l O CH H
O MgBr 2 O
CH 2 CHCH SnBu 3 + CH O C N Ph O N Ph
2
2
3
OMOM O 82%
OMOM
13 m OTBDMS
O CH
TBDMSO CH 3 + O MgBr 2 CH 3 H O
CH O C N Ph O Ph
SnBu 3 3 2 N
CH 3 O
CH 3 78%
a. M. Koreeda and Y. Tanaka, Chem. Lett., 1297 (1982).
b. V. Peruzzo and G. Tagliavini, J. Organomet. Chem., 162, 37 (1978).
c. A. Gambaro, V. Peruzzo, G. Plazzogna, and G. Tagliavini, J. Organomet. Chem., 197, 45 (1980).
d. L. A. Paquette and G. D. Maynard, J. Am. Chem. Soc., 114, 5018 (1992).
e. D.-P. Quintard, B. Elissondo, and M. Pereyre, J. Org. Chem., 48, 1559 (1983).
f. G. E. Keck and E. P. Boden, Tetrahedron Lett., 25, 1879 (1984).
g. T. Harada and T. Mukaiyama, Chem. Lett., 1109 (1981).
h. K. Maruyama, Y. Ishiara, and Y. Yamamoto, Tetrahedron Lett., 22, 4235 (1981).
i. L. A. Paquette and P. C. Astles, J. Org. Chem., 58, 165 (1993).
j. J. A. Marshall, S. Beaudoin, and K. Lewinski, J. Org. Chem. 58, 5876 (1993).
k. H. Nagaoka, and Y. Kishi, Tetrahedron, 37, 3873 (1981).
l. K.-Y. Lee, C.-Y. Oh, Y.-H. Kim, J. E. Joo, and W.-H. Ham, Tetrahedron Lett., 43, 9361 (2002).
m. K.-Y. Lee, C.-Y. Oh, and W.-H. Ham, Org. Lett., 4, 4403 (2002).
BF should involve an open TS. The observed stereochemistry is syn but the approach
3
is anti-Felkin.
SnBu
3
H
CH 3 H CH
CH 3 CH H H 3
H 3 CH 3 CH CH CH 3
3 3 O O
O H
F B HO
3
H H H CH 3 O C
3
CH CH 3
OH 3
CH O C CH 3 CH O C CH 3
3
2
3
2
Entry 9 was used in the synthesis of a furanocembranolide. This reaction
presumably proceeds through a trichlorostannane intermediate and involves allylic