Page 877 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 877
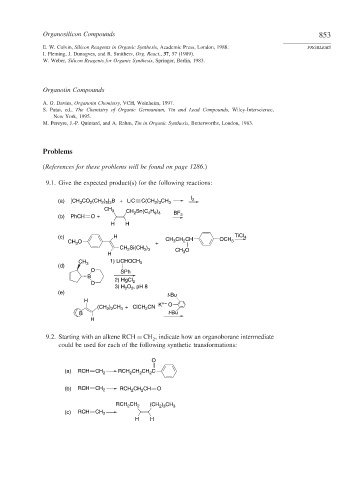
Organosilicon Compounds 853
E. W. Colvin, Silicon Reagents in Organic Synthesis, Academic Press, London, 1988. PROBLEMS
I. Fleming, J. Dunogves, and R. Smithers, Org. React., 37, 57 (1989).
W. Weber, Silicon Reagents for Organic Synthesis, Springer, Berlin, 1983.
Organotin Compounds
A. G. Davies, Organotin Chemistry, VCH, Weinheim, 1997.
S. Patai, ed., The Chemistry of Organic Germanium, Tin and Lead Compounds, Wiley-Interscience,
New York, 1995.
M. Pereyre, J.-P. Quintard, and A. Rahm, Tin in Organic Synthesis, Butterworths, London, 1983.
Problems
(References for these problems will be found on page 1286.)
9.1. Give the expected product(s) for the following reactions:
I
) CH
(a) [CH CO (CH ) ] B + LiC C(CH 2 3 3 2
3
2
2 5 3
CH 3 CH 2 Sn(C H )
(b) PhCH O + 4 9 3 BF 3
H H
(c) H CH CH CH OCH TiCl 4
CH O + 3 2 3
3
CH Si(CH ) CH O
3 3
2
H 3
CH 1) LiCHOCH 3
(d) 3
O SPh
B
O 2) HgCl 2
3) H O , pH 8
2
2
(e)
t-Bu
H + –
(CH ) CH + ClCH CN K O
3
2
2 2
B t-Bu
H
9.2. Starting with an alkene RCH = CH , indicate how an organoborane intermediate
2
could be used for each of the following synthetic transformations:
O
(a) RCH CH 2 RCH CH CH C
2
2
2
(b) RCH CH 2 RCH 2 CH CH O
2
) CH
RCH CH 2 (CH 2 3 3
2
(c) RCH CH 2
H H