Page 875 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 875
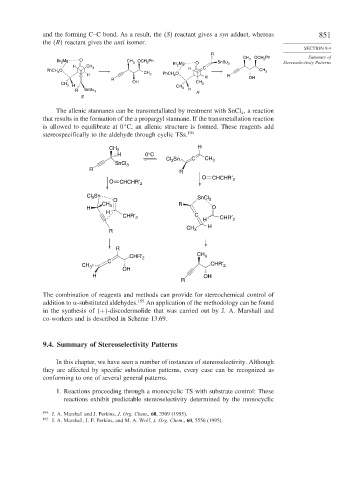
and the forming C–C bond. As a result, the S reactant gives a syn adduct, whereas 851
the R reactant gives the anti isomer.
SECTION 9.4
R
CH OCH Ph Summary of
Br Mg O CH OCH Ph SnBu 3 2
2 3 2 Br Mg O 3 Stereoselectivity Patterns
H CH 2 C
PhCH O 3 CH H CH 3
2
2
C H 3 PhCH O H R OH
R
CH OH CH 3
3 H CH
R SnBu 3 H
3
R
S
The allenic stannanes can be transmetallated by treatment with SnCl , a reaction
4
that results in the formation of the a propargyl stannane. If the transmetallation reaction
is allowed to equilibrate at 0 C, an allenic structure is formed. These reagents add
stereospecifically to the aldehyde through cyclic TSs. 194
CH 3 H
H 0°C
Cl Sn C CH 3
SnCl 3 3
R R
O CHCHR′
O CHCHR′ 2 2
Sn
Cl 3
O SnCl 3
CH 3 R
H O
H
CHR′ C CHR′
2
H 2
CH H
R 3
R
CHR′ 2 CH 3
C CHR′
CH 3 2
OH
H OH
R
The combination of reagents and methods can provide for stereochemical control of
addition to -substituted aldehydes. 195 An application of the methodology can be found
in the synthesis of + -discodermolide that was carried out by J. A. Marshall and
co-workers and is described in Scheme 13.69.
9.4. Summary of Stereoselectivity Patterns
In this chapter, we have seen a number of instances of stereoselectivity. Although
they are affected by specific substitution patterns, every case can be recognized as
conforming to one of several general patterns.
1. Reactions proceeding through a monocyclic TS with substrate control: These
reactions exhibit predictable stereoselectivity determined by the monocyclic
194 J. A. Marshall and J. Perkins, J. Org. Chem., 60, 3509 (1995).
195
J. A. Marshall, J. F. Perkins, and M. A. Wolf, J. Org. Chem., 60, 5556 (1995).