Page 878 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 878
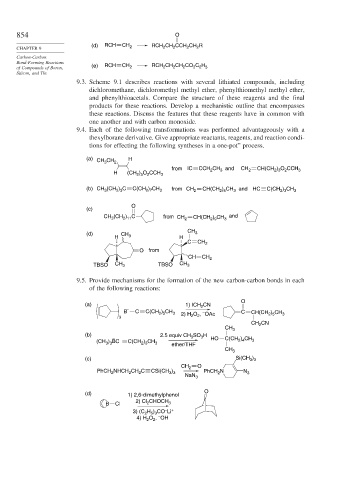
854 O
(d) RCH CH RCH 2 CH 2 CCH 2 CH 2 R
CHAPTER 9 2
Carbon-Carbon
Bond-Forming Reactions
2
2
2 2 5
of Compounds of Boron, (e) RCH CH 2 RCH 2 CH CH CO C H
Silicon, and Tin
9.3. Scheme 9.1 describes reactions with several lithiated compounds, including
dichloromethane, dichloromethyl methyl ether, phenylthiomethyl methyl ether,
and phenylthioacetals. Compare the structure of these reagents and the final
products for these reactions. Develop a mechanistic outline that encompasses
these reactions. Discuss the features that these reagents have in common with
one another and with carbon monoxide.
9.4. Each of the following transformations was performed advantageously with a
thexylborane derivative. Give appropriate reactants, reagents, and reaction condi-
tions for effecting the following syntheses in a one-pot” process.
(a) CH CH 2 H
3
from IC CCH CH and CH 2 CH(CH ) O CCH 3
2
3
2
2 3
H (CH 2 ) 5 O 2 CCH 3
(b) CH (CH ) C C(CH ) CH 3 from CH 2 CH(CH ) CH 3 and HC C(CH ) CH 3
3
2 3
2 7
2 3
2 5
O
(c)
(CH ) C and
CH 3 2 11 from CH 2 CH(CH ) CH 3
2 9
CH
(d) CH 3
H 3 H
C CH 2
O from
CH CH 2
TBSO CH 3 TBSO CH 3
9.5. Provide mechanisms for the formation of the new carbon-carbon bonds in each
of the following reactions:
O
(a) CN
_ 1) ICH 2
B C C(CH ) CH 3 – C CH(CH 2 5
) CH
2 5
2
3 2) H 2 O , OAc 3
CH 2 CN
CH 3
(b) 2.5 equiv CH SO H
– 3 3 HO C(CH ) CH
(CH ) BC C(CH ) CH 3 ether/ THF 2 4 3
2 3
3 3
CH 3
)
(c) Si(CH 3 3
CH 2 O
PhCH NHCH CH C CSi(CH ) PhCH N N 3
2
2
2
3 3
2
NaN 3
(d) 1) 2,6-dimethylphenol O
2) Cl CHOCH
B Cl 2 3
–
3) (C H ) CO Li +
2 5 3
–
4) H O , OH
2
2