Page 883 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 883
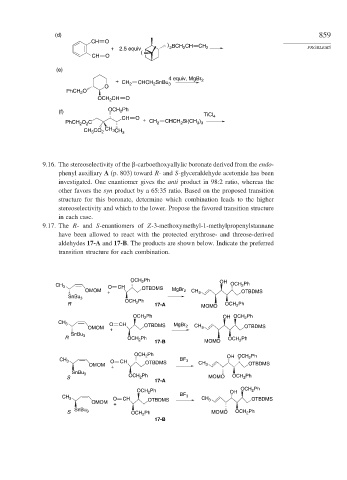
(d) 859
CH O
) BCH CH CH
+ 2.5 equiv 2 2 2 PROBLEMS
(
CH O
(e)
+ CH 2 CHCH 2 SnBu 3 4 equiv, MgBr 2
O
PhCH O
2
CH O
OCH 2
(f) OCH Ph
2
TiCl
CH O 4
PhCH O C + CH 2 CHCH Si(CH )
3 3
2
2
2
CH CO 2 CH 3 CH 3
3
9.16. The stereoselectivity of the -carboethoxyallylic boronate derived from the endo-
phenyl auxiliary A (p. 803) toward R- and S-glyceraldehyde acetonide has been
investigated. One enantiomer gives the anti product in 98:2 ratio, whereas the
other favors the syn product by a 65:35 ratio. Based on the proposed transition
structure for this boronate, determine which combination leads to the higher
stereoselectivity and which to the lower. Propose the favored transition structure
in each case.
9.17. The R- and S-enantiomers of Z-3-methoxymethyl-1-methylpropenylstannane
have been allowed to react with the protected erythrose- and threose-derived
aldehydes 17-A and 17-B. The products are shown below. Indicate the preferred
transition structure for each combination.
OCH 2 Ph OH
CH 3 O CH OTBDMS OCH 2 Ph
OMOM + MgBr 2 CH 3 OTBDMS
SnBu 3
OCH 2 Ph
R 17-A MOMO OCH 2 Ph
OCH 2 Ph OH OCH 2 Ph
CH 3 O CH OTBDMS MgBr 2
OMOM + CH 3 OTBDMS
SnBu 3
R OCH 2 Ph OCH 2 Ph
17-B MOMO
OCH 2 Ph OH OCH 2 Ph
CH 3 O CH OTBDMS BF 3
OMOM + CH 3 OTBDMS
SnBu 3
S OCH 2 Ph MOMO OCH 2 Ph
17-A
OCH 2 Ph OH OCH 2 Ph
BF 3
CH 3 O CH OTBDMS CH 3 OTBDMS
OMOM +
S SnBu 3 OCH 2 Ph MOMO OCH 2 Ph
17-B