Page 882 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 882
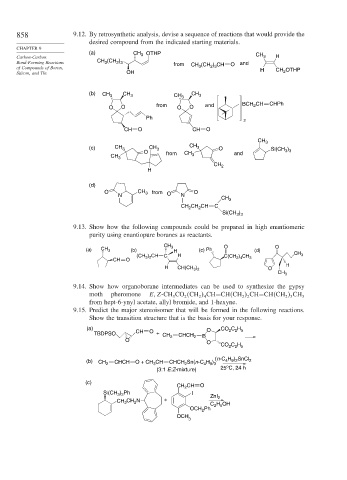
858 9.12. By retrosynthetic analysis, devise a sequence of reactions that would provide the
desired compound from the indicated starting materials.
CHAPTER 9
(a) CH OTHP
Carbon-Carbon 3 CH 3 H
3
Bond-Forming Reactions CH (CH ) from CH (CH ) CH O and
2 3
of Compounds of Boron, 3 2 3 H CH OTHP
Silicon, and Tin OH 2
(b) CH 3 CH 3 CH 3 CH 3
2
O O from O O and BCH CH CHPh
Ph
2
CH O CH O
CH 3
(c) CH 3 CH 3 CH 3 O Si(CH )
O from CH and 3 3
CH 3 3
CH 2
H
(d)
O CH 3 from O O
N N
CH 3
2
CH 2 CH CH C
Si(CH )
3 3
9.13. Show how the following compounds could be prepared in high enantiomeric
purity using enantiopure boranes as reactants.
CH 3 O O
(a) CH 3 (b) H (c) Ph (d)
(CH 3 ) 2 CH C H CH 3
CH O C(CH 2 ) 4 CH 3
H
H CH(CH 3 ) 2 O
CH 3
9.14. Show how organoborane intermediates can be used to synthesize the gypsy
moth pheromone E
Z-CH CO CH CH=CH CH CH=CH CH CH
3 2 2 4 2 2 2 3 3
from hept-6-ynyl acetate, allyl bromide, and 1-hexyne.
9.15. Predict the major stereoisomer that will be formed in the following reactions.
Show the transition structure that is the basis for your response.
(a) O CO C H
2 2 5
TBDPSO CH O + CH 2 CHCH 2 B
O
O
CO C H
2 2 5
(n-C H ) SnCl
(b) CH 2 CHCH O + CH CH CHCH 2 Sn(n-C H ) 4 9 2 2
3
4 9 3
ο
(3:1 E:Z-mixture) 25 C, 24 h
(c)
CH CH O
2
Si(CH ) Ph I ZnI
3 2
CH CH N + 2
2
2
H OH
C 2 5
Ph
OCH 2
OCH
3