Page 881 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 881
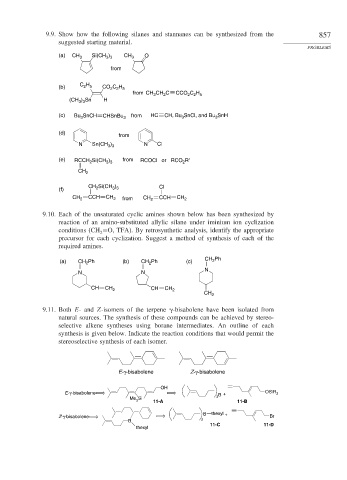
9.9. Show how the following silanes and stannanes can be synthesized from the 857
suggested starting material.
PROBLEMS
(a) CH 3 Si(CH ) CH 3 O
3 3
from
C H
2 5
(b) C H CO 2 2 5
from CH CH C CCO 2 2 5
C H
3
2
(CH ) Sn H
3 3
(c) Bu 3 SnCH CHSnBu 3 from HC CH, Bu 3 SnCl, and Bu SnH
3
(d)
from
N Sn(CH 3 3 N Cl
)
(e) RCCH 2 Si(CH ) from RCOCl or RCO R′
3 3
2
CH 2
CH Si(CH ) Cl
(f) 2 3 3
CCH CH CCH CH
CH 2 2 from CH 2 2
9.10. Each of the unsaturated cyclic amines shown below has been synthesized by
reaction of an amino-substituted allylic silane under iminium ion cyclization
conditions (CH =O, TFA). By retrosynthetic analysis, identify the appropriate
2
precursor for each cyclization. Suggest a method of synthesis of each of the
required amines.
CH Ph
(a) CH 2 Ph (b) CH Ph (c) 2
2
N
N N
CH CH 2 CH CH 2
CH 2
9.11. Both E- and Z-isomers of the terpene -bisabolene have been isolated from
natural sources. The synthesis of these compounds can be achieved by stereo-
selective alkene syntheses using borane intermediates. An outline of each
synthesis is given below. Indicate the reaction conditions that would permit the
stereoselective synthesis of each isomer.
E-γ-bisabolene Z-γ-bisabolene
OH
E-γ-bisabolene B + OSiR 3
Me Si 11-A 3 11-B
3
B thexyl +
Z-γ-bisabolene Br
B 2
11-C 11-D
thexyl