Page 888 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 888
864 10.1.1.2. Polyene Cyclization. Perhaps the most synthetically useful of the carbo-
cation alkylation reactions is the cyclization of polyenes having two or more double
CHAPTER 10 bonds positioned in such a way that successive bond-forming steps can occur. This
Reactions Involving process, called polyene cyclization, has proven to be an effective way of making
Carbocations, Carbenes,
and Radicals as Reactive polycyclic compounds containing six-membered and, in some cases, five-membered
Intermediates rings. The reaction proceeds through an electrophilic attack and requires that the
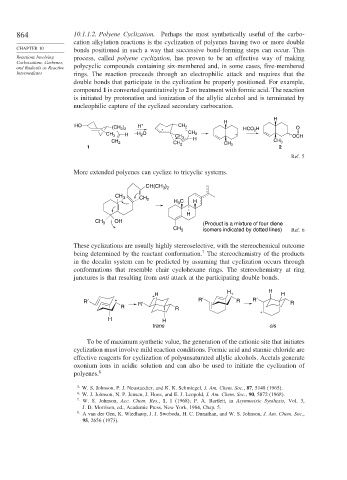
double bonds that participate in the cyclization be properly positioned. For example,
compound 1 is converted quantitatively to 2 on treatment with formic acid. The reaction
is initiated by protonation and ionization of the allylic alcohol and is terminated by
nucleophilic capture of the cyclized secondary carbocation.
H
H
HO H + CH 2
(CH 2 ) 2 HCO 2 H O
+
H –H 2 O CH 2 +
CH 3
CH 3 OCH
H
CH 2 CH 3
CH 2 CH 3
1 2
Ref. 5
More extended polyenes can cyclize to tricyclic systems.
CH(CH )
3 2
CH 3 CH
2
H C H
3
H
CH 3 OH (Product is a mixture of four diene
CH 3 isomers indicated by dotted lines) Ref. 6
These cyclizations are usually highly stereoselective, with the stereochemical outcome
7
being determined by the reactant conformation. The stereochemistry of the products
in the decalin system can be predicted by assuming that cyclization occurs through
conformations that resemble chair cyclohexane rings. The stereochemistry at ring
junctures is that resulting from anti attack at the participating double bonds.
H H
H + H
+
R′ + R′ R′ R R′ R
R R
+
H H
trans cis
To be of maximum synthetic value, the generation of the cationic site that initiates
cyclization must involve mild reaction conditions. Formic acid and stannic chloride are
effective reagents for cyclization of polyunsaturated allylic alcohols. Acetals generate
oxonium ions in acidic solution and can also be used to initiate the cyclization of
polyenes. 8
5 W. S. Johnson, P. J. Neustaedter, and K. K. Schmiegel, J. Am. Chem. Soc., 87, 5148 (1965).
6
W. J. Johnson, N. P. Jensen, J. Hooz, and E. J. Leopold, J. Am. Chem. Soc., 90, 5872 (1968).
7 W. S. Johnson, Acc. Chem. Res., 1, 1 (1968); P. A. Bartlett, in Asymmetric Synthesis, Vol. 3,
J. D. Morrison, ed., Academic Press, New York, 1984, Chap. 5.
8
A van der Gen, K. Wiedhaup, J. J. Swoboda, H. C. Dunathan, and W. S. Johnson, J. Am. Chem. Soc.,
95, 2656 (1973).