Page 890 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 890
866
X
CHAPTER 10 1) i PrOTiCl 3 H
Reactions Involving 2) HCl
Carbocations, Carbenes, HO
and Radicals as Reactive H
Intermediates
O 2a X = H
)
2b X = Si(CH 3 3
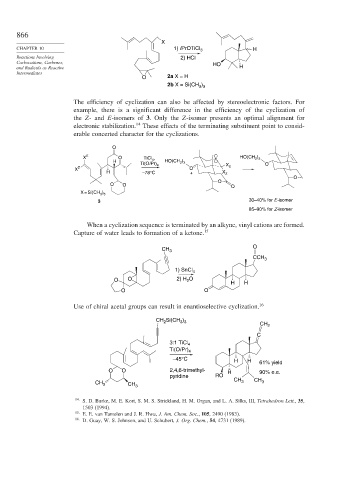
The efficiency of cyclization can also be affected by stereoelectronic factors. For
example, there is a significant difference in the efficiency of the cyclization of
the Z- and E-isomers of 3. Only the Z-isomer presents an optimal alignment for
electronic stabilization. 14 These effects of the terminating substituent point to consid-
erable concerted character for the cyclizations.
O
)
X E O TiCl , HO(CH 2 3
)
H 4 HO(CH 2 3
Ti(Oi Pr) 4 X O
X Z O E
H –78°C + X Z
O
O
O O O
X = Si(CH 3 ) 3
3 30–40% for E-isomer
85–90% for Z-isomer
When a cyclization sequence is terminated by an alkyne, vinyl cations are formed.
Capture of water leads to formation of a ketone. 15
CH 3 O
CCH 3
1) SnCl 4
O O 2) H O
2
H H
O O
Use of chiral acetal groups can result in enantioselective cyclization. 16
CH Si(CH )
3 3
2
CH 2
C
3:1 TiCl 4
Ti(Oi Pr) 4
–45°C H H 61% yield
O O 2,4,6-trimethyl- H 90% e.e.
pyridine RO
CH 3 CH 3
CH 3 CH 3
14 S. D. Burke, M. E. Kort, S. M. S. Strickland, H. M. Organ, and L. A. Silks, III, Tetrahedron Lett., 35,
1503 (1994).
15 E. E. van Tamelen and J. R. Hwu, J. Am. Chem. Soc., 105, 2490 (1983).
16
D. Guay, W. S. Johnson, and U. Schubert, J. Org. Chem., 54, 4731 (1989).