Page 889 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 889
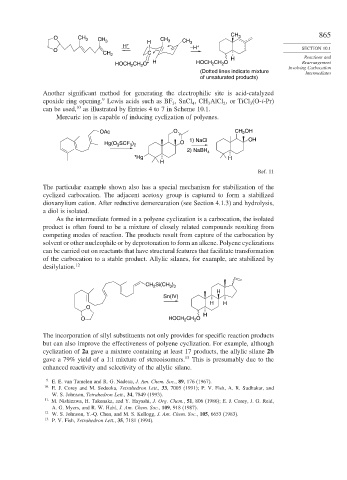
CH 865
O CH 3 CH 3 CH 3 CH 3
H + H 3 –H +
O SECTION 10.1
CH 2 C
H Reactions and
HOCH CH O + H HOCH 2 CH O Rearrangement
2
2
2
Involving Carbocation
(Dotted lines indicate mixture Intermediates
of unsaturated products)
Another significant method for generating the electrophilic site is acid-catalyzed
9
epoxide ring opening. Lewis acids such as BF , SnCl ,CH AlCl , or TiCl (O-i-Pr)
3
2
4
3
3
can be used, 10 as illustrated by Entries 4 to 7 in Scheme 10.1.
Mercuric ion is capable of inducing cyclization of polyenes.
OAc O CH 2 OH
+
1) NaCl OH
Hg(O SCF ) O
3 2
3
2) NaBH 4
+
Hg H
H
Ref. 11
The particular example shown also has a special mechanism for stabilization of the
cyclized carbocation. The adjacent acetoxy group is captured to form a stabilized
dioxanylium cation. After reductive demercuration (see Section 4.1.3) and hydrolysis,
a diol is isolated.
As the intermediate formed in a polyene cyclization is a carbocation, the isolated
product is often found to be a mixture of closely related compounds resulting from
competing modes of reaction. The products result from capture of the carbocation by
solvent or other nucleophile or by deprotonation to form an alkene. Polyene cyclizations
can be carried out on reactants that have structural features that facilitate transformation
of the carbocation to a stable product. Allylic silanes, for example, are stabilized by
desilylation. 12
CH Si(CH )
3 3
2
H
Sn(IV)
H H
O
H
O HOCH 2 CH O
2
The incorporation of silyl substituents not only provides for specific reaction products
but can also improve the effectiveness of polyene cyclization. For example, although
cyclization of 2a gave a mixture containing at least 17 products, the allylic silane 2b
gave a 79% yield of a 1:l mixture of stereoisomers. 13 This is presumably due to the
enhanced reactivity and selectivity of the allylic silane.
9 E. E. van Tamelen and R. G. Nadeau, J. Am. Chem. Soc., 89, 176 (1967).
10 E. J. Corey and M. Sodeoka, Tetrahedron Lett., 33, 7005 (1991); P. V. Fish, A. R. Sudhakar, and
W. S. Johnson, Tetrahedron Lett., 34, 7849 (1993).
11
M. Nishizawa, H. Takenaka, and Y. Hayashi, J. Org. Chem., 51, 806 (1986); E. J. Corey, J. G. Reid,
A. G. Myers, and R. W. Hahl, J. Am. Chem. Soc., 109, 918 (1987).
12 W. S. Johnson, Y.-Q. Chen, and M. S. Kellogg, J. Am. Chem. Soc., 105, 6653 (1983).
13
P. V. Fish, Tetrahedron Lett., 35, 7181 (1994).