Page 891 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 891
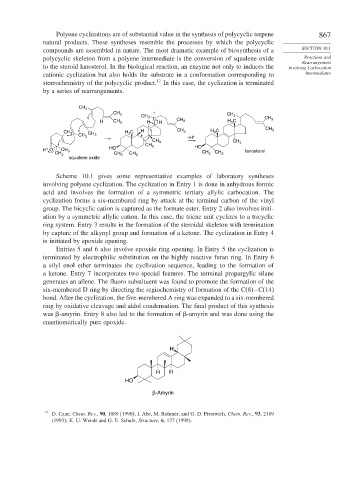
Polyene cyclizations are of substantial value in the synthesis of polycyclic terpene 867
natural products. These syntheses resemble the processes by which the polycyclic
compounds are assembled in nature. The most dramatic example of biosynthesis of a SECTION 10.1
polycyclic skeleton from a polyene intermediate is the conversion of squalene oxide Reactions and
Rearrangement
to the steroid lanosterol. In the biological reaction, an enzyme not only to induces the Involving Carbocation
cationic cyclization but also holds the substrate in a conformation corresponding to Intermediates
stereochemistry of the polycyclic product. 17 In this case, the cyclization is terminated
by a series of rearrangements.
CH 3
+
CH 3 CH 3
CH 3
H CH 3 H C H CH 3 H 3 C CH 3
H 3 C H CH 3 H 3 C CH 3
CH 3
CH 3
CH 3 +
–H
CH 3 CH 3
CH 3 HO
H + O CH 3 HO lanosterol
CH 3 CH 3
CH 3 CH 3 CH 3
squalene oxide
Scheme 10.1 gives some representative examples of laboratory syntheses
involving polyene cyclization. The cyclization in Entry 1 is done in anhydrous formic
acid and involves the formation of a symmetric tertiary allylic carbocation. The
cyclization forms a six-membered ring by attack at the terminal carbon of the vinyl
group. The bicyclic cation is captured as the formate ester. Entry 2 also involves initi-
ation by a symmetric allylic cation. In this case, the triene unit cyclizes to a tricyclic
ring system. Entry 3 results in the formation of the steroidal skeleton with termination
by capture of the alkynyl group and formation of a ketone. The cyclization in Entry 4
is initiated by epoxide opening.
Entries 5 and 6 also involve epoxide ring opening. In Entry 5 the cyclization is
terminated by electrophilic substitution on the highly reactive furan ring. In Entry 6
a silyl enol ether terminates the cyclization sequence, leading to the formation of
a ketone. Entry 7 incorporates two special features. The terminal propargylic silane
generates an allene. The fluoro substituent was found to promote the formation of the
six-membered D ring by directing the regiochemistry of formation of the C(8)−C(14)
bond. After the cyclization, the five-membered A ring was expanded to a six-membered
ring by oxidative cleavage and aldol condensation. The final product of this synthesis
was -amyrin. Entry 8 also led to the formation of -amyrin and was done using the
enantiomerically pure epoxide.
H
H H
HO
β-Amyrin
17
D. Cane, Chem. Rev., 90, 1089 (1990); I. Abe, M. Rohmer, and G. D. Prestwich, Chem. Rev., 93, 2189
(1993); K. U. Wendt and G. E. Schulz, Structure, 6, 127 (1998).