Page 896 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 896
872 X X
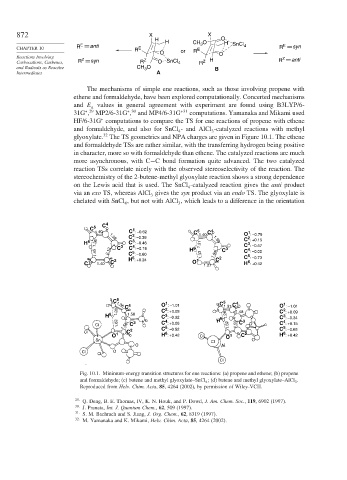
H H CH O O H SnCl
CHAPTER 10 R E anti R E E 3 4 R E syn
O or R O
Reactions Involving Z Z anti
Carbocations, Carbenes, R syn R Z O SnCl 4 R Z H R
and Radicals as Reactive CH O B
3
Intermediates A
The mechanisms of simple ene reactions, such as those involving propene with
ethene and formaldehyde, have been explored computationally. Concerted mechanisms
and E values in general agreement with experiment are found using B3LYP/6-
a
∗ 30
∗ 29
31G , MP2/6-31G , and MP4/6-31G ∗31 computations. Yamanaka and Mikami used
∗
HF/6-31G computations to compare the TS for ene reactions of propene with ethene
and formaldehyde, and also for SnCl - and AlCl -catalyzed reactions with methyl
4 3
32
glyoxylate. The TS geometries and NPA charges are given in Figure 10.1. The ethene
and formaldehyde TSs are rather similar, with the transferring hydrogen being positive
in character, more so with formaldehyde than ethene. The catalyzed reactions are much
more asynchronous, with C−C bond formation quite advanced. The two catalyzed
reaction TSs correlate nicely with the observed stereoselectivity of the reaction. The
stereochemistry of the 2-butene-methyl glyoxylate reaction shows a strong dependence
on the Lewis acid that is used. The SnCl -catalyzed reaction gives the anti product
4
via an exo TS, whereas AlCl gives the syn product via an endo TS. The glyoxylate is
3
chelated with SnCl , but not with AlCl , which leads to a difference in the orientation
4 3
C 4
C 5 1
1.40 C : –0.62 C 5 C 4 1
2 1.40 O : –0.79
C : –0.39 1.39 2
1.38
3
H 6 1.36 C 3 C : –0.46 6 1.31 C : +0.15
3
C : –0.57
4
4
1.45 2.12 C : –0.19 H 1.33 1.94 2 C 3 C : –0.00
5
C : –0.60
5
C : –0.73
6
6
C 1 1.40 C 2 H : +0.24 O 1 1.27 C H : +0.42
5
1 C 5 C 4 1
C 1.36
C 4 O : –1.01 1.37 O : –1.01
2
1.28 C : +0.08 1.48 C : +0.09
2
3
3
H 6 1.50 C : –0.32 6 1.28 C : –0.34
1.62 3 C : +0.06 H C 3 C : +0.15
4
4
Cl 1.58 C 5 1.52 1.59 O 5
Cl C 2 C : –0.52 2 C : –0.65
6
6
O 1 H : +0.42 Cl O 1 C H : +0.42
Sn Cl O
O Al
Cl O
Cl
Cl
Fig. 10.1. Minimum-energy transition structures for ene reactions: (a) propene and ethene; (b) propene
and formaldehyde; (c) butene and methyl glyoxylate–SnCl 4 ; (d) butene and methyl glyoxylate–AlCl 3 .
Reproduced from Helv. Chim. Acta, 85, 4264 (2002), by permission of Wiley-VCH.
29
Q. Deng, B. E. Thomas, IV, K. N. Houk, and P. Dowd, J. Am. Chem. Soc., 119, 6902 (1997).
30
J. Pranata, Int. J. Quantum Chem., 62, 509 (1997).
31 S. M. Bachrach and S. Jiang, J. Org. Chem., 62, 8319 (1997).
32
M. Yamanaka and K. Mikami, Helv. Chim. Acta, 85, 4264 (2002).