Page 901 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 901
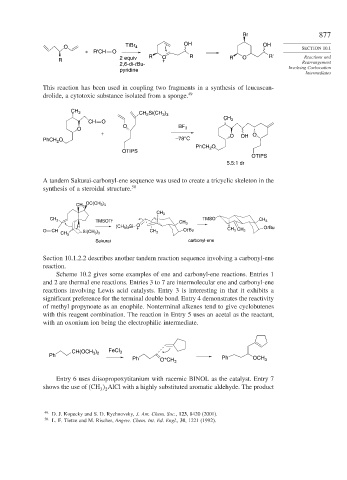
Br 877
OH OH
O TiBr 4 SECTION 10.1
+ R'CH O
Reactions and
R 2 equiv R O R R O R' Rearrangement
+
2,6-di-t Bu-
pyridine Involving Carbocation
Intermediates
This reaction has been used in coupling two fragments in a synthesis of leucascan-
drolide, a cytotoxic substance isolated from a sponge. 49
CH 3
CH 2 Si(CH 3 ) 3
CH 3
CH O
O
O BF 3
+ O OH O
PhCH 2 O –78°C
PhCH 2 O
OTIPS
OTIPS
5.5:1 dr
A tandem Sakurai-carbonyl-ene sequence was used to create a tricyclic skeleton in the
synthesis of a steroidal structure. 50
OC(CH 3 ) 3
CH 3
CH 3
TMSO
TMSOTf CH 3
CH 3 CH 3
H (CH 3 ) 3 Si O Ot Bu
+
O CH CH 3 Ot Bu CH 3 CH 2
CH 3 Si(CH 3 ) 3
Sakurai carbonyl-ene
Section 10.1.2.2 describes another tandem reaction sequence involving a carbonyl-ene
reaction.
Scheme 10.2 gives some examples of ene and carbonyl-ene reactions. Entries 1
and 2 are thermal ene reactions. Entries 3 to 7 are intermolecular ene and carbonyl-ene
reactions involving Lewis acid catalysts. Entry 3 is interesting in that it exhibits a
significant preference for the terminal double bond. Entry 4 demonstrates the reactivity
of methyl propynoate as an enophile. Nonterminal alkenes tend to give cyclobutenes
with this reagent combination. The reaction in Entry 5 uses an acetal as the reactant,
with an oxonium ion being the electrophilic intermediate.
) FeCl
CH(OCH 3 2 3
Ph
+
Ph O CH 3 Ph OCH 3
Entry 6 uses diisopropoxytitanium with racemic BINOL as the catalyst. Entry 7
shows the use of (CH AlCl with a highly substituted aromatic aldehyde. The product
3 2
49 D. J. Kopecky and S. D. Rychnovsky, J. Am. Chem. Soc., 123, 8420 (2001).
50
L. F. Tietze and M. Rischer, Angew. Chem. Int. Ed. Engl., 31, 1221 (1992).