Page 904 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 904
880 Scheme 10.2. (Continued)
CHAPTER 10 a. C. S. Rondestvedt, Jr., Org. Synth., IV, 766 (1963).
b. P. Beak, Z. Song, and J. E. Resek, J. Org. Chem., 57, 944 (1992).
Reactions Involving c. A. T. Blomquist and R. J. Himics, J. Org. Chem., 33, 1156 (1968).
Carbocations, Carbenes, d. B. B. Snider, D. J. Rodini, R. S. E. Conn, and S. Sealfon, J. Am. Chem. Soc., 101, 5283 (1979).
and Radicals as Reactive e. A. Ladepeche, E. Tam, J.-E. Arcel, and L. Ghosez, Synthesis, 1375 (2004).
Intermediates f. M. A. Brimble and M. K. Edmonds, Synth. Commun., 26, 243 (1996).
g. M. Majewski and G. W. Bantle, Synth. Commun., 20, 2549 (1990); M. Majewski, N. M. Irvine, and G. W. Bantle,
J. Org. Chem., 59, 6697 (1994).
h. W. Oppolzer, K. K. Mahalanabis, and K. Battig, Helv. Chim. Acta, 60, 2388 (1977).
i. W. Oppolzer and C. Robbiani, Helv. Chim. Acta, 63, 2010 (1980).
j. T. K. Sarkar, B. K. Ghorai, S. K. Nandy, B. Mukherjee, and A. Banerji, J. Org. Chem., 62, 6006 (1997).
k. V. K. Aggarwal, G. P Vennall, P. N. Davey, and C. Newman, Tetrahedron Lett., 39, 1997 (1998).
l. L. F. Courtney, M. Lange, M. R. Uskokovics, and P. M. Wovkulich, Tetrahedron Lett., 39, 3363 (1998).
m. J.-M. Weibel and D. Heissler, Synlett, 391 (1993).
n. B. B. Snider, N. H. Vo, and S. V. O’Neill, J. Org. Chem., 63, 4732 (1998).
o. J. A. Marshall and M. W. Andersen, J. Org. Chem., 57, 5851 (1992).
p. M. Terada and K. Mikami, J. Chem. Soc., Chem. Commun., 833 (1994).
q. W. H. Miles, E. J. Fialcowitz, and E. S. Halstead, Tetrahedron, 57, 9925 (2001).
r. D. A. Evans, S. W. Tregay, C. S. Burgey, N. A. Paras, and T. Vojkovsky, J. Am. Chem. Soc., 122, 7936 (2000).
s. K. Mikami, A. Yoshida, and Y. Matsumoto, Tetrahedron Lett., 37, 8515 (1996).
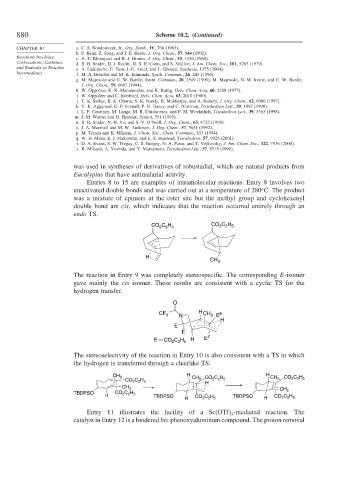
was used in syntheses of derivatives of robustadial, which are natural products from
Eucalyptus that have antimalarial activity.
Entries 8 to 15 are examples of intramolecular reactions. Entry 8 involves two
unactivated double bonds and was carried out at a temperature of 280 C. The product
was a mixture of epimers at the ester site but the methyl group and cyclohexenyl
double bond are cis, which indicates that the reaction occurred entirely through an
endo TS.
CO C H CO C H
2 2 5
2 2 5
H
CH 3
The reaction in Entry 9 was completely stereospecific. The corresponding E-isomer
gave mainly the cis isomer. These results are consistent with a cyclic TS for the
hydrogen transfer.
O
CF 3 N H CH 3 E E
H
E
E
E CO C H H E Z
2 2 5
The stereoselectivity of the reaction in Entry 10 is also consistent with a TS in which
the hydrogen is transferred through a chairlike TS.
H H
CH 3
CH 3 CO 2 C 2 H 5 CH 3 CO 2 C 2 H 5
CO 2 C 2 H 5
H
CH 3
CH 2
TBDPSO CO 2 C 2 H 5
H TBDPSO TBDPSO CO 2 C 2 H 5
H CO 2 C 2 H 5 H
Entry 11 illustrates the facility of a Sc(OTf) -mediated reaction. The
3
catalyst in Entry 12 is a hindered bis-phenoxyaluminum compound. The proton removal