Page 907 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 907
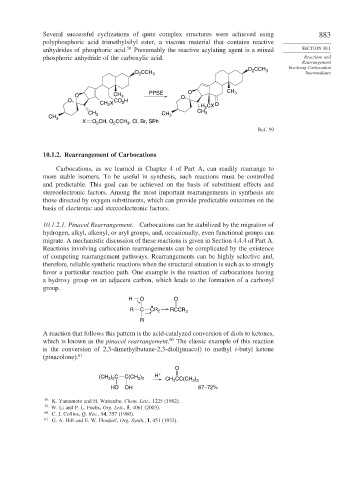
Several successful cyclizations of quite complex structures were achieved using 883
polyphosphoric acid trimethylsilyl ester, a viscous material that contains reactive
anhydrides of phosphoric acid. 58 Presumably the reactive acylating agent is a mixed SECTION 10.1
phosphoric anhydride of the carboxylic acid. Reactions and
Rearrangement
O CCH Involving Carbocation
O CCH 3 2 3 Intermediates
2
O CH 3 PPSE O O CH 3
O CO 2 H
CH X H CX O
2
2
CH CH CH 3
CH 3 3 3
X O CH, O CCH , Cl, Br, SPh
3
2
2
Ref. 59
10.1.2. Rearrangement of Carbocations
Carbocations, as we learned in Chapter 4 of Part A, can readily rearrange to
more stable isomers. To be useful in synthesis, such reactions must be controlled
and predictable. This goal can be achieved on the basis of substituent effects and
stereoelectronic factors. Among the most important rearrangements in synthesis are
those directed by oxygen substituents, which can provide predictable outcomes on the
basis of electronic and stereoelectronic factors.
10.1.2.1. Pinacol Rearrangement. Carbocations can be stabilized by the migration of
hydrogen, alkyl, alkenyl, or aryl groups, and, occasionally, even functional groups can
migrate. A mechanistic discussion of these reactions is given in Section 4.4.4 of Part A.
Reactions involving carbocation rearrangements can be complicated by the existence
of competing rearrangement pathways. Rearrangements can be highly selective and,
therefore, reliable synthetic reactions when the structural situation is such as to strongly
favor a particular reaction path. One example is the reaction of carbocations having
a hydroxy group on an adjacent carbon, which leads to the formation of a carbonyl
group.
H O O
+
R C CR 2 RCCR 3
R
A reaction that follows this pattern is the acid-catalyzed conversion of diols to ketones,
which is known as the pinacol rearrangement. 60 The classic example of this reaction
is the conversion of 2,3-dimethylbutane-2,3-diol(pinacol) to methyl t-butyl ketone
(pinacolone). 61
O
(CH ) C C(CH ) H + CH CC(CH )
3 2
3 2
3
3 3
HO OH 67–72%
58
K. Yamamoto and H. Watanabe, Chem. Lett., 1225 (1982).
59 W. Li and P. L. Fuchs, Org. Lett., 5, 4061 (2003).
60 C. J. Collins, Q. Rev., 14, 357 (1960).
61
G. A. Hill and E. W. Flosdorf, Org. Synth., I, 451 (1932).