Page 912 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 912
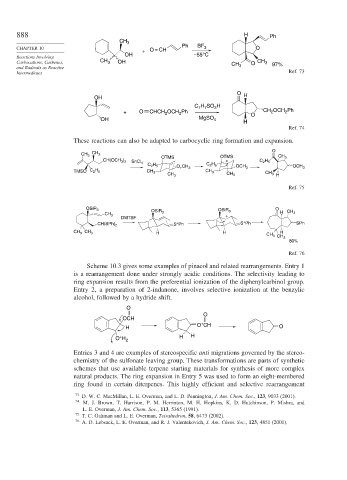
888 H Ph
CH 3
CHAPTER 10 O = CH Ph BF 3 O
+
OH –55°C
Reactions Involving
Carbocations, Carbenes, CH 3 OH CH O CH 3 97%
and Radicals as Reactive 3
Intermediates Ref. 73
O
OH H
H SO H
C 7 7 3
+ O CHCH 2 OCH 2 Ph O CH 2 OCH Ph
2
OH MgSO 4 H
Ref. 74
These reactions can also be adapted to carbocyclic ring formation and expansion.
O
CH 3
CH 3
OTMS OTMS CH 3
CH(OCH 3 ) 2 + C 2 H 5
SnCl 4
C 2 H 5 C 2 H 5
O + CH 3 OCH 3 OCH 3
TMSO C 2 H 5 CH 3 CH 3
CH 3 CH 3 CH 3 H
Ref. 75
OSiR 3 O
OSiR 3 OSiR 3 H CH 3
CH 3 +
DMTSF
+
+
CH(SPh) 2 S Ph S Ph SPh
CH 3 CH 3 H H H
CH 3 CH 3
80%
Ref. 76
Scheme 10.3 gives some examples of pinacol and related rearrangements. Entry 1
is a rearrangement done under strongly acidic conditions. The selectivity leading to
ring expansion results from the preferential ionization of the diphenylcarbinol group.
Entry 2, a preparation of 2-indanone, involves selective ionization at the benzylic
alcohol, followed by a hydride shift.
O
O
OCH
+
O CH
H O
+
O H 2 H H
Entries 3 and 4 are examples of stereospecific anti migrations governed by the stereo-
chemistry of the sulfonate leaving group. These transformations are parts of synthetic
schemes that use available terpene starting materials for synthesis of more complex
natural products. The ring expansion in Entry 5 was used to form an eight-membered
ring found in certain diterpenes. This highly efficient and selective rearrangement
73 D. W. C. MacMillan, L. E. Overman, and L. D. Pennington, J. Am. Chem. Soc., 123, 9033 (2001).
74 M. J. Brown, T. Harrison, P. M. Herrinton, M. H. Hopkins, K. D. Hutchinson, P. Mishra, and
L. E. Overman, J. Am. Chem. Soc., 113, 5365 (1991).
75
T. C. Gahman and L. E. Overman, Tetrahedron, 58, 6473 (2002).
76 A. D. Lebsack, L. E. Overman, and R. J. Valentekovich, J. Am. Chem. Soc., 123, 4851 (2001).