Page 917 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 917
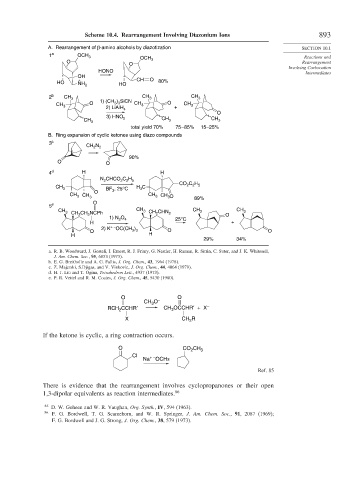
Scheme 10.4. Rearrangement Involving Diazonium Ions 893
A. Rearrangement of β-amino alcohols by diazotization SECTION 10.1
1 a OCH 3
OCH Reactions and
O 3 Rearrangement
O
Involving Carbocation
HONO Intermediates
OH
+ CH O 80%
HO
NH 3 HO
2 b CH 3 CH 3 CH 3
1) (CH ) SiCN
3 3
CH 3 O 2) LiAlH CH 3 O + CH 3
4
O
3) HNO 2 CH CH
CH 3 3
3
total yield 70% 75 – 85% 15–25%
B. Ring expansion of cyclic ketones using diazo compounds
3 c
N
CH 2 2
90%
O O
4 d H H
N CHCO C H
2
2 2 5
C H
CO 2 2 5
CH 3 BF , 25°C H C
3
O 3
CH 3 CH 3 CH 3 CH 3O 89%
O
5 e
CH CH CH 3 CH 3
3 CH 2 CH NCPh 3 CH 2 CHN 2 O
2
1) N O 4 25°C
2
H +
+ –
2) K OC(CH )
O 3 3 O O
H H
29% 34%
a. R. B. Woodward, J. Gosteli, I. Ernest, R. J. Friary, G. Nestler, H. Raman, R. Sitrin, C. Suter, and J. K. Whitesell,
J. Am. Chem. Soc., 95, 6853 (1973).
b. E. G. Breitholle and A. G. Fallis, J. Org. Chem., 43, 1964 (1978).
c. Z. Majerski, S.Djigas, and V. Vinkovic, J. Org. Chem., 44, 4064 (1979).
d. H. J. Liu and T. Ogina, Tetrahedron Lett., 4937 (1973).
e. P. R. Vettel and R. M. Coates, J. Org. Chem., 45, 5430 (1980).
O O
CH O –
3
RCH CCHR′ CH OCCHR′ + X –
3
2
X CH 2 R
If the ketone is cyclic, a ring contraction occurs.
O CO 2 CH 3
Cl
Na + – OCH3
Ref. 85
There is evidence that the rearrangement involves cyclopropanones or their open
1,3-dipolar equivalents as reaction intermediates. 86
85 D. W. Goheen and W. R. Vaughan, Org. Synth., IV, 594 (1963).
86
F. G. Bordwell, T. G. Scamehorn, and W. R. Springer, J. Am. Chem. Soc., 91, 2087 (1969);
F. G. Bordwell and J. G. Strong, J. Org. Chem., 38, 579 (1973).