Page 921 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 921
CO 2 C(CH 3 ) 3 897
C(CH )
CO 2 3 3
CO 2 C(CH 3 ) 3 N
N SECTION 10.1
N
Reactions and
KOt Bu
1) NCS Rearrangement
Involving Carbocation
2) MCPBA Intermediates
Cl S 58% 97%
S
O O
Ref. 94
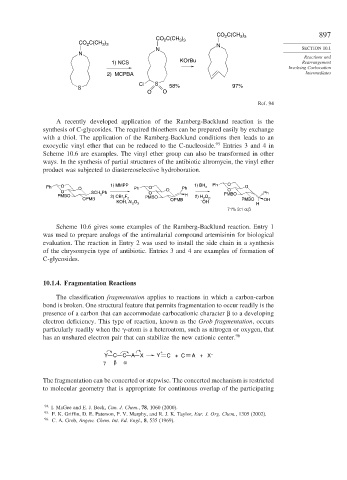
A recently developed application of the Ramberg-Backlund reaction is the
synthesis of C-glycosides. The required thioethers can be prepared easily by exchange
with a thiol. The application of the Ramberg-Backlund conditions then leads to an
exocyclic vinyl ether that can be reduced to the C-nucleoside. 95 Entries 3 and 4 in
Scheme 10.6 are examples. The vinyl ether group can also be transformed in other
ways. In the synthesis of partial structures of the antibiotic altromycin, the vinyl ether
product was subjected to diastereoselective hydroboration.
Ph O O 1) MMPP Ph O Ph 1) BH 3 Ph O O
O Ph O O O Ph
PMBO SCH 2 2) CBr F PMBO H 2) H O , PMBO
OPMB 2 2 OPMB 2 2 PMBO OH
KOH, Al O – OH
2 3 H
71% 3:1 α:β
Scheme 10.6 gives some examples of the Ramberg-Backlund reaction. Entry 1
was used to prepare analogs of the antimalarial compound artemisinin for biological
evaluation. The reaction in Entry 2 was used to install the side chain in a synthesis
of the chrysomycin type of antibiotic. Entries 3 and 4 are examples of formation of
C-glycosides.
10.1.4. Fragmentation Reactions
The classification fragmentation applies to reactions in which a carbon-carbon
bond is broken. One structural feature that permits fragmentation to occur readily is the
presence of a carbon that can accommodate carbocationic character to a developing
electron deficiency. This type of reaction, known as the Grob fragmentation, occurs
particularly readily when the -atom is a heteroatom, such as nitrogen or oxygen, that
has an unshared electron pair that can stabilize the new cationic center. 96
+
Y C C A X Y C + C A + X –
γ β α
The fragmentation can be concerted or stepwise. The concerted mechanism is restricted
to molecular geometry that is appropriate for continuous overlap of the participating
94 I. MaGee and E. J. Beck, Can. J. Chem., 78, 1060 (2000).
95 F. K. Griffin, D. E. Paterson, P. V. Murphy, and R. J. K. Taylor, Eur. J. Org. Chem., 1305 (2002).
96
C. A. Grob, Angew. Chem. Int. Ed. Engl., 8, 535 (1969).