Page 924 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 924
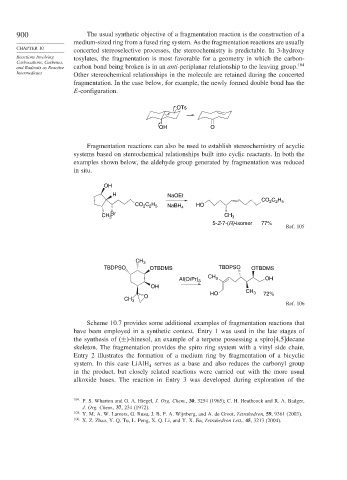
900 The usual synthetic objective of a fragmentation reaction is the construction of a
medium-sized ring from a fused ring system. As the fragmentation reactions are usually
CHAPTER 10
concerted stereoselective processes, the stereochemistry is predictable. In 3-hydroxy
Reactions Involving tosylates, the fragmentation is most favorable for a geometry in which the carbon-
Carbocations, Carbenes, 104
and Radicals as Reactive carbon bond being broken is in an anti-periplanar relationship to the leaving group.
Intermediates Other stereochemical relationships in the molecule are retained during the concerted
fragmentation. In the case below, for example, the newly formed double bond has the
E-configuration.
OTs
OH O
Fragmentation reactions can also be used to establish stereochemistry of acyclic
systems based on stereochemical relationships built into cyclic reactants. In both the
examples shown below, the aldehyde group generated by fragmentation was reduced
in situ.
OH
H NaOEt
CO C H
2 2 5
CO C H NaBH 4 HO
2 2 5
CH 3 Br CH 3
5-Z-7-(R)-isomer 77%
Ref. 105
CH 3
TBDPSO OTBDMS TBDPSO OTBDMS
Al(Oi Pr) 3 CH 3 OH
OH
HO CH 3 72%
O
CH 3
Ref. 106
Scheme 10.7 provides some additional examples of fragmentation reactions that
have been employed in a synthetic context. Entry 1 was used in the late stages of
the synthesis of (± -hinesol, an example of a terpene possessing a spiro[4,5]decane
skeleton. The fragmentation provides the spiro ring system with a vinyl side chain.
Entry 2 illustrates the formation of a medium ring by fragmentation of a bicyclic
system. In this case LiAlH serves as a base and also reduces the carbonyl group
4
in the product, but closely related reactions were carried out with the more usual
alkoxide bases. The reaction in Entry 3 was developed during exploration of the
104 P. S. Wharton and G. A. Hiegel, J. Org. Chem., 30, 3254 (1965); C. H. Heathcock and R. A. Badger,
J. Org. Chem., 37, 234 (1972).
105 Y. M. A. W. Lamers, G. Rusu, J. B. P. A. Wijnberg, and A. de Groot, Tetrahedron, 59, 9361 (2003).
106
X. Z. Zhao, Y. Q. Tu, L. Peng, X. Q. Li, and Y. X. Jia, Tetrahedron Lett., 45, 3213 (2004).