Page 928 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 928
904 triplets. Substituents that act as electron-pair donors stabilize the singlet state more
than the triplet state by delocalization of an electron pair into the empty p orbital. 108
CHAPTER 10
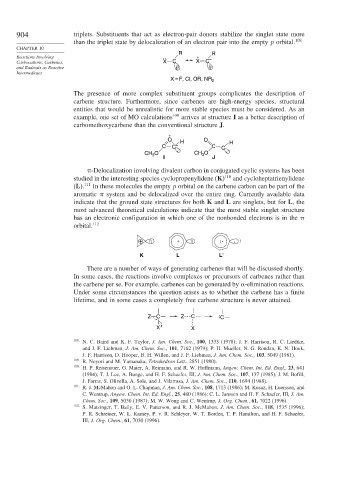
R R
Reactions Involving : + –
Carbocations, Carbenes, X C X C
and Radicals as Reactive
Intermediates
X = F, Cl, OR, NR 2
The presence of more complex substituent groups complicates the description of
carbene structure. Furthermore, since carbenes are high-energy species, structural
entities that would be unrealistic for more stable species must be considered. As an
example, one set of MO calculations 109 arrives at structure I as a better description of
carbomethoxycarbene than the conventional structure J.
+
O H O H
C C – C C
O O
CH 3 CH 3
I J
-Delocalization involving divalent carbon in conjugated cyclic systems has been
studied in the interesting species cyclopropenylidene (K) 110 and cycloheptatrienylidene
(L). 111 In these molecules the empty p orbital on the carbene carbon can be part of the
aromatic system and be delocalized over the entire ring. Currently available data
indicate that the ground state structures for both K and L are singlets, but for L, the
most advanced theoretical calculations indicate that the most stable singlet structure
has an electronic configuration in which one of the nonbonded electrons is in the
orbital. 112
+ + . .
K L L'
There are a number of ways of generating carbenes that will be discussed shortly.
In some cases, the reactions involve complexes or precursors of carbenes rather than
the carbene per se. For example, carbenes can be generated by -elimination reactions.
Under some circumstances the question arises as to whether the carbene has a finite
lifetime, and in some cases a completely free carbene structure is never attained.
Z C Z C : C
X X
108
N. C. Baird and K. F. Taylor, J. Am. Chem. Soc., 100, 1333 (1978); J. F. Harrison, R. C. Liedtke,
and J. F. Liebman, J. Am. Chem. Soc., 101, 7162 (1979); P. H. Mueller, N. G. Rondan, K. N. Houk,
J. F. Harrison, D. Hooper, B. H. Willen, and J. F. Liebman, J. Am. Chem. Soc., 103, 5049 (1981).
109 R. Noyori and M. Yamanaka, Tetrahedron Lett., 2851 (1980).
110
H. P. Reisenauer, G. Maier, A. Reimann, and R. W. Hoffmann, Angew. Chem. Int. Ed. Engl., 23, 641
(1984); T. J. Lee, A. Bunge, and H. F. Schaefer, III, J. Am. Chem. Soc., 107, 137 (1985); J. M. Bofill,
J. Farras, S. Olivella, A. Sole, and J. Vilarrasa, J. Am. Chem. Soc., 110, 1694 (1988).
111 R. J. McMahon and O. L. Chapman, J. Am. Chem. Soc., 108, 1713 (1986); M. Kusaz, H. Luerssen, and
C. Wentrup, Angew. Chem. Int. Ed. Engl., 25, 480 (1986); C. L. Janssen and H. F. Schaefer, III, J. Am.
Chem. Soc., 109, 5030 (1987); M. W. Wong and C. Wentrup, J. Org. Chem., 61, 7022 (1996).
112
S. Matzinger, T. Bally, E. V. Patterson, and R. J. McMahon, J. Am. Chem. Soc., 118, 1535 (1996);
P. R. Schreiner, W. L. Karney, P. v. R. Schleyer, W. T. Borden, T. P. Hamilton, and H. F. Schaefer,
III, J. Org. Chem., 61, 7030 (1996).