Page 927 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 927
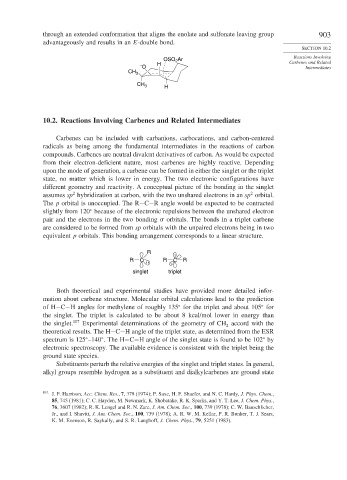
through an extended conformation that aligns the enolate and sulfonate leaving group 903
advantageously and results in an E-double bond.
SECTION 10.2
Reactions Involving
OSO Ar Carbenes and Related
2
– O H Intermediates
CH 3
CH 3 H
10.2. Reactions Involving Carbenes and Related Intermediates
Carbenes can be included with carbanions, carbocations, and carbon-centered
radicals as being among the fundamental intermediates in the reactions of carbon
compounds. Carbenes are neutral divalent derivatives of carbon. As would be expected
from their electron-deficient nature, most carbenes are highly reactive. Depending
upon the mode of generation, a carbene can be formed in either the singlet or the triplet
state, no matter which is lower in energy. The two electronic configurations have
different geometry and reactivity. A conceptual picture of the bonding in the singlet
2
2
assumes sp hybridization at carbon, with the two unshared electrons in an sp orbital.
The p orbital is unoccupied. The R−C−R angle would be expected to be contracted
slightly from 120 because of the electronic repulsions between the unshared electron
pair and the electrons in the two bonding orbitals. The bonds in a triplet carbene
are considered to be formed from sp orbitals with the unpaired electrons being in two
equivalent p orbitals. This bonding arrangement corresponds to a linear structure.
R
R C R C R
singlet triplet
Both theoretical and experimental studies have provided more detailed infor-
mation about carbene structure. Molecular orbital calculations lead to the prediction
of H−C−H angles for methylene of roughly 135 for the triplet and about 105 for
the singlet. The triplet is calculated to be about 8 kcal/mol lower in energy than
the singlet. 107 Experimental determinations of the geometry of CH accord with the
2
theoretical results. The H−C−H angle of the triplet state, as determined from the ESR
spectrum is 125 –140 . The H−C−H angle of the singlet state is found to be 102 by
electronic spectroscopy. The available evidence is consistent with the triplet being the
ground state species.
Substituents perturb the relative energies of the singlet and triplet states. In general,
alkyl groups resemble hydrogen as a substituent and dialkylcarbenes are ground state
107
J. F. Harrison, Acc. Chem. Res., 7, 378 (1974); P. Saxe, H. F. Shaefer, and N. C. Hardy, J. Phys. Chem.,
85, 745 (1981); C. C. Hayden, M. Newmark, K. Shobatake, R. K. Sparks, and Y. T. Lee, J. Chem. Phys.,
76, 3607 (1982); R. K. Lengel and R. N. Zare, J. Am. Chem. Soc., 100, 739 (1978); C. W. Bauschlicher,
Jr., and I. Shavitt, J. Am. Chem. Soc., 100, 739 (1978); A. R. W. M. Kellar, P. R. Bunker, T. J. Sears,
K. M. Evenson, R. Saykally, and S. R. Langhoff, J. Chem. Phys., 79, 5251 (1983).