Page 923 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 923
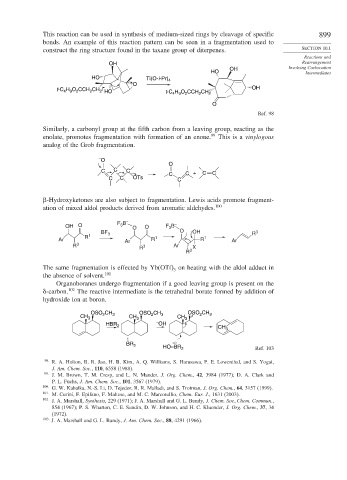
This reaction can be used in synthesis of medium-sized rings by cleavage of specific 899
bonds. An example of this reaction pattern can be seen in a fragmentation used to
construct the ring structure found in the taxane group of diterpenes. SECTION 10.1
Reactions and
OH Rearrangement
OH Involving Carbocation
HO Intermediates
HO Ti(O-i-Pr) 4
O
OH
t-C H 9 O 2 CCH 2 CH 2 HO t-C 4 9 2 2 2
4
H O CCH CH
O
Ref. 98
Similarly, a carbonyl group at the fifth carbon from a leaving group, reacting as the
enolate, promotes fragmentation with formation of an enone. 99 This is a vinylogous
analog of the Grob fragmentation.
– O
O
C C C C C + C C
C C OTs C
-Hydroxyketones are also subject to fragmentation. Lewis acids promote fragment-
ation of mixed aldol products derived from aromatic aldehydes. 100
OH O F B – O O F 3 B –
3
BF 3 O OH R 3
R 1 1 1
Ar Ar R R Ar
R 3 3 Ar
R X
R 3
The same fragmentation is effected by Yb(OTf) on heating with the aldol adduct in
3
the absence of solvent. 101
Organoboranes undergo fragmentation if a good leaving group is present on the
-carbon. 102 The reactive intermediate is the tetrahedral borate formed by addition of
hydroxide ion at boron.
OSO CH 3 OSO CH OSO CH 3
2
2
CH 3 CH 3 2 3 CH 3
HBR 2 – OH CH 3
–
BR 2 HO–BR 2 Ref. 103
98
R. A. Holton, R. R. Juo, H. B. Kim, A. Q. Williams, S. Harusawa, P. E. Lowenthal, and S. Yogai,
J. Am. Chem. Soc., 110, 6558 (1988).
99 J. M. Brown, T. M. Cresp, and L. N. Mander, J. Org. Chem., 42, 3984 (1977); D. A. Clark and
P. L. Fuchs, J. Am. Chem. Soc., 101, 3567 (1979).
100 G. W. Kabalka, N.-S. Li, D. Tejedor, R. R. Malladi, and S. Trotman, J. Org. Chem., 64, 3157 (1999).
101
M. Curini, F. Epifano, F. Maltese, and M. C. Marcotullio, Chem. Eur. J., 1631 (2003).
102 J. A. Marshall, Synthesis, 229 (1971); J. A. Marshall and G. L. Bundy, J. Chem. Soc.,Chem. Commun.,
854 (1967); P. S. Wharton, C. E. Sundin, D. W. Johnson, and H. C. Kluender, J. Org. Chem., 37,34
(1972).
103
J. A. Marshall and G. L. Bundy, J. Am. Chem. Soc., 88, 4291 (1966).