Page 918 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 918
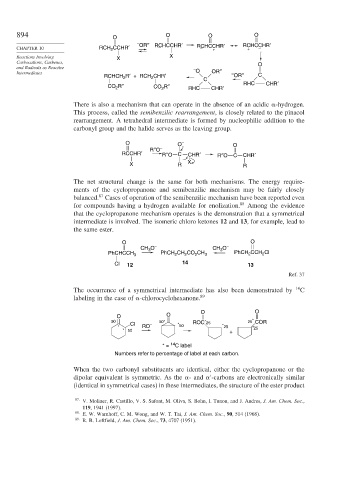
894 O O O O
– OR″ RCHCCHR′ RCHCCHR′ RCHCCHR′
CHAPTER 10 RCH 2 CCHR′ – – + + –
Reactions Involving X X
Carbocations, Carbenes, O
and Radicals as Reactive –
Intermediates O OR″ –
RCHCH R′ + RCH CHR′ C OR″ C
2
2
CO 2 R″ CO 2 R″ RHC CHR′ RHC CHR′
There is also a mechanism that can operate in the absence of an acidic -hydrogen.
This process, called the semibenzilic rearrangement, is closely related to the pinacol
rearrangement. A tetrahedral intermediate is formed by nucleophilic addition to the
carbonyl group and the halide serves as the leaving group.
O O – O
R″O –
RCCHR′ R″O C CHR′ R″O C CHR′
X R X R
The net structural change is the same for both mechanisms. The energy require-
ments of the cyclopropanone and semibenzilic mechanism may be fairly closely
87
balanced. Cases of operation of the semibenzilic mechanism have been reported even
for compounds having a hydrogen available for enolization. 88 Among the evidence
that the cyclopropanone mechanism operates is the demonstration that a symmetrical
intermediate is involved. The isomeric chloro ketones 12 and 13, for example, lead to
the same ester.
O O
CH O – CH O –
3
3
PhCHCCH 3 PhCH CH CO CH 3 PhCH CCH Cl
2
2
2
2
2
Cl 12 14 13
Ref. 37
The occurrence of a symmetrical intermediate has also been demonstrated by 14 C
labeling in the case of -chlorocyclohexanone. 89
O O
O O
50 50* ROC 25 25 * COR
*
* Cl RO – * 50 * 25 * 25
* 50
+
14
* = C label
Numbers refer to percentage of label at each carbon.
When the two carbonyl substituents are identical, either the cyclopropanone or the
dipolar equivalent is symmetric. As the - and -carbons are electronically similar
(identical in symmetrical cases) in these intermediates, the structure of the ester product
87
V. Moliner, R. Castillo, V. S. Safont, M. Oliva, S. Bohn, I. Tunon, and J. Andres, J. Am. Chem. Soc.,
119, 1941 (1997).
88 E. W. Warnhoff, C. M. Wong, and W. T. Tai, J. Am. Chem. Soc., 90, 514 (1968).
89
R. B. Loftfield, J. Am. Chem. Soc., 73, 4707 (1951).