Page 914 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 914
890 Scheme 10.3. (Continued)
CHAPTER 10 a. H. E. Zaugg, M. Freifelder, and B. W. Horrom, J. Org. Chem., 15, 1191 (1950).
b. J. E. Horan and R. W. Schliessler, Org. Synth., 41, 53 (1961).
Reactions Involving c. G. Buchi, W. Hofheinz, and J. V. Paukstelis, J. Am. Chem. Soc., 91, 6473 (1969).
Carbocations, Carbenes, d. D. F. MacSweeney and R. Ramage, Tetrahedron, 27, 1481 (1971).
and Radicals as Reactive e. P. Magnus, C. Diorazio, T. J. Donohoe, M. Giles, P. Pye, J. Tarrant, and S. Thom, Tetrahedron, 52,
Intermediates 14147 (1996).
f. Y. Kita, Y. Yoshida, S. Mihara, D.-F. Fang, K. Higuchi, A. Furukawa, and H. Fujioka, Tetrahedron
Lett., 38, 8315 (1997).
g. J. H. Rigby and K. R. Fales, Tetrahedron Lett., 39, 1525 (1998).
h. K. D. Eom, J. V. Raman, H. Kim, and J. K. Cha, J. Am. Chem. Soc., 125, 5415 (2003).
i. H. Arimoto, K. Nishimura, M. Kuramoto, and D. Uemura, Tetrahedron Lett., 39, 9513 (1998).
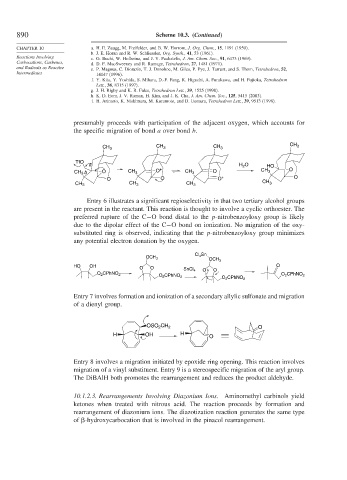
presumably proceeds with participation of the adjacent oxygen, which accounts for
the specific migration of bond a over bond b.
CH 3
CH 3 CH 3 CH 3
TfO
a H 2 O HO
CH 3 b O CH 3 O + CH 3 O CH 3 O
O O O + O
CH 3
CH 3 CH 3 CH 3
Entry 6 illustrates a significant regioselectivity in that two tertiary alcohol groups
are present in the reactant. This reaction is thought to involve a cyclic orthoester. The
preferred rupture of the C−O bond distal to the p-nitrobenzoyloxy group is likely
due to the dipolar effect of the C−O bond on ionization. No migration of the oxy-
substituted ring is observed, indicating that the p-nitrobenzoyloxy group minimizes
any potential electron donation by the oxygen.
Cl 4 Sn
OCH 3
OCH 3
HO OH O
O O
SnCl 4 O O
O 2 CPhNO 2 O 2 CPhNO 2
O 2 CPhNO 2
O 2 CPhNO 2
Entry 7 involves formation and ionization of a secondary allylic sulfonate and migration
of a dienyl group.
CH
OSO 2 2 O
H OH H O
Entry 8 involves a migration initiated by epoxide ring opening. This reaction involves
migration of a vinyl substituent. Entry 9 is a stereospecific migration of the aryl group.
The DiBAlH both promotes the rearrangement and reduces the product aldehyde.
10.1.2.3. Rearrangements Involving Diazonium Ions. Aminomethyl carbinols yield
ketones when treated with nitrous acid. The reaction proceeds by formation and
rearrangement of diazonium ions. The diazotization reaction generates the same type
of -hydroxycarbocation that is involved in the pinacol rearrangement.