Page 920 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 920
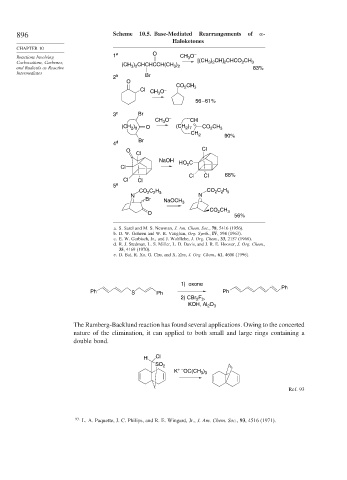
896 Scheme 10.5. Base-Mediated Rearrangements of -
Haloketones
CHAPTER 10
Reactions Involving 1 a O CH O –
3
) CH] CHCO CH
Carbocations, Carbenes, (CH ) CHCHCCH(CH ) [(CH 3 2 2 2 3
and Radicals as Reactive 3 2 3 2 83%
Intermediates Br
2 b
O
CO 2 CH 3
Cl CH O –
3
56 – 61%
3 c Br
CH O – CH
3
(CH ) O (CH ) CO CH 3
2 7
2 8
2
CH 2 90%
Br
4 d
O Cl
Cl
NaOH
HO 2 C
Cl
Cl Cl 68%
Cl Cl
5 e
CO C H CO C H
2 2 5
2 2 5
N N
Br NaOCH 3
CH
CO 2 3
O
56%
a. S. Sarel and M. S. Newman, J. Am. Chem. Soc., 78, 5416 (1956).
b. D. W. Goheen and W. R. Vaughan, Org. Synth., IV, 594 (1963).
c. E. W. Garbisch, Jr., and J. Wohllebe, J. Org. Chem., 33, 2157 (1968).
d. R. J. Stedman, L. S. Miller, L. D. Davis, and J. R. E. Hoover, J. Org. Chem.,
35, 4169 (1970).
e. D. Bai, R. Xu, G. Chu, and X. Zhu, J. Org. Chem., 61, 4600 (1996).
1) oxone
Ph
Ph S Ph Ph
2) CBr F ,
2 2
KOH, Al O 3
2
The Ramberg-Backlund reaction has found several applications. Owing to the concerted
nature of the elimination, it can applied to both small and large rings containing a
double bond.
H Cl
SO 2
K + – OC(CH )
3 3
Ref. 93
93
L. A. Paquette, J. C. Philips, and R. E. Wingard, Jr., J. Am. Chem. Soc., 93, 4516 (1971).