Page 903 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 903
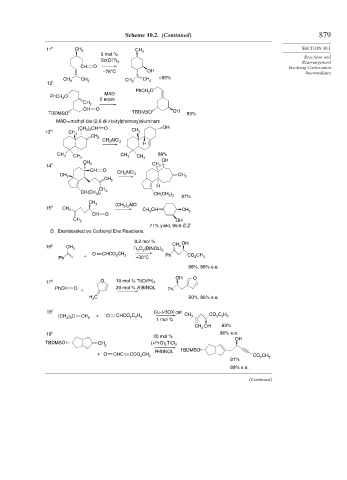
Scheme 10.2. (Continued) 879
11 k CH 3 CH SECTION 10.1
5 mol % 3
Reactions and
Sc(OTf) 3 Rearrangement
CH O Involving Carbocation
–78°C OH
Intermediates
CH >95%
CH 3 CH CH 2
12 l 3 3
PhCH O
MAD 2
O
PhCH 2
2 equiv
CH 2
CH O
TBDMSO TBDMSO OH 83%
MAD = methyl-bis-(2,6-di-t-butylphenoxy)aluminum
(CH ) CH O CH OH
13 m CH 2 2 3
3
CH
2
CH AlCl
3 2
H
CH 3 CH 3 CH 3 CH 3 89%
CH OH
14 n 3 CH 3
CH O AlCl
CH CH 3 2 CH
3 2
CH
2
H
CH
CH(CH ) 3 )
3 2
CH(CH 3 2
87%
CH 3 (CH ) AlCl
15 o CH 3 3 2 CH 3 CH CH
CH O 3
CH OH
2
71% yield, 95:5 E:Z
D. Enantioselective Carbonyl Ene Reactions.
0.2 mol % OH
16 p CH CH 2
3 Ti O (BINOL) 2
2
2
O C CH
+ HCO 2 3 Ph CO CH
Ph –30°C 2 3
88%, 99% e.e.
OH O
17 q O 10 mol % Ti(Oi Pr) 4
PhCH O + 20 mol % S-BINOL Ph
C 90%, 95% e.e.
H 2
18 r Cu-t-BOX cat
(CH 3 ) 2 C CH 2 + O CHCO C 2 H 5 CH 3 CO C H 5
2
2
2
1 mol %
CH OH 83%
2
19 s 96% e.e.
20 mol %
OH
TBDMSO CH (i-PrO) TiCl
2 2 2
TBDMSO
R-BINOL
+ O CHC CCO CH 3 CO 2 CH 3
2
81%
89% e.e.
(Continued)