Page 897 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 897
of the unshared electrons on the ester oxygen. The exo TS is believed to be favored 873
by an electrostatic interaction between the oxygen and C(4).
SECTION 10.1
CH 3 Reactions and
CH 3 CH 3 H CH 3 Rearrangement
H AlCl 3 + SnCl 4 H H Involving Carbocation
H CO CH 3 Intermediates
2
O O
H O CHCO 2 CH 3 O OCH 3
Cl 3 Al Cl Sn
4
OH OH
CH 3
H CO CH 3 CO CH 3 CH 3
2
2
H H
HO CO CH 3 CH 3 CH 3
2
H HO CO 2 CH 3
syn anti
Despite the cyclic character of these TSs, both the bond distances and charge distri-
bution are characteristic of a high degree of charge separation, with the butenyl
fragment assuming the character of an allylic carbocation.
Visual models, additional information and exercises on the Carbonyl-Ene
Reaction can be found in the Digital Resource available at: Springer.com/carey-
sundberg.
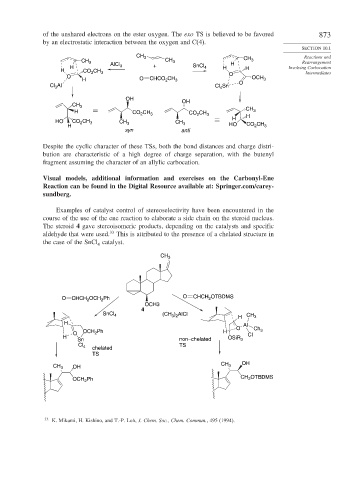
Examples of catalyst control of stereoselectivity have been encountered in the
course of the use of the ene reaction to elaborate a side chain on the steroid nucleus.
The steroid 4 gave stereoisomeric products, depending on the catalysts and specific
aldehyde that were used. 33 This is attributed to the presence of a chelated structure in
the case of the SnCl catalyst.
4
CH 3
O CHCH OCH Ph O CHCH 2 OTBDMS
2
2
OCH3
4
SnCl 4 (CH ) AlCl H CH 3
3 2
H Al
O Ch 3
O OCH 2 Ph H Cl
H
Sn non–chelated OSiR 3
Cl 4 chelated TS
TS
CH 3 OH CH 3 OH
OCH Ph CH OTBDMS
2
2
33
K. Mikami, H. Kishino, and T.-P. Loh, J. Chem. Soc., Chem. Commun., 495 (1994).