Page 894 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 894
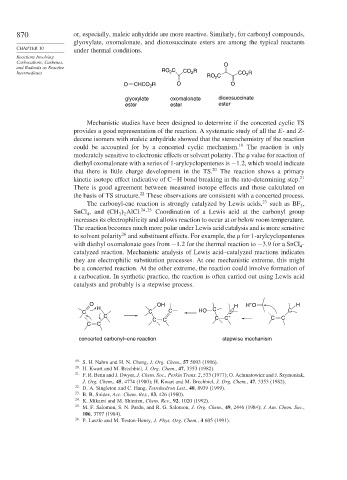
870 or, especially, maleic anhydride are more reactive. Similarly, for carbonyl compounds,
glyoxylate, oxomalonate, and dioxosuccinate esters are among the typical reactants
CHAPTER 10
under thermal conditions.
Reactions Involving
Carbocations, Carbenes, O
and Radicals as Reactive RO C
2
Intermediates 2 CO R CO 2 R
RO C
2
O CHCO 2 R O O
glyoxylate oxomalonate dioxosuccinate
ester ester ester
Mechanistic studies have been designed to determine if the concerted cyclic TS
provides a good representation of the reaction. A systematic study of all the E- and Z-
decene isomers with maleic anhydride showed that the stereochemistry of the reaction
could be accounted for by a concerted cyclic mechanism. 19 The reaction is only
moderately sensitive to electronic effects or solvent polarity. The value for reaction of
diethyl oxomalonate with a series of 1-arylcyclopentenes is −1 2, which would indicate
that there is little charge development in the TS. 20 The reaction shows a primary
kinetic isotope effect indicative of C−H bond breaking in the rate-determining step. 21
There is good agreement between measured isotope effects and those calculated on
22
the basis of TS structure. These observations are consistent with a concerted process.
The carbonyl-ene reaction is strongly catalyzed by Lewis acids, 23 such as BF ,
3
SnCl , and (CH AlCl. 24
25 Coordination of a Lewis acid at the carbonyl group
3 2
4
increases its electrophilicity and allows reaction to occur at or below room temperature.
The reaction becomes much more polar under Lewis acid catalysis and is more sensitive
26
to solvent polarity and substituent effects. For example, the for 1-arylcyclopentenes
with diethyl oxomalonate goes from −1 2 for the thermal reaction to −3 9 for a SnCl -
4
catalyzed reaction. Mechanistic analysis of Lewis acid–catalyzed reactions indicates
they are electrophilic substitution processes. At one mechanistic extreme, this might
be a concerted reaction. At the other extreme, the reaction could involve formation of
a carbocation. In synthetic practice, the reaction is often carried out using Lewis acid
catalysts and probably is a stepwise process.
O OH H H O H
+
H C
C C C HO C
C +
C C C C C C
C C
concerted carbonyl–ene reaction stepwise mechanism
19 S. H. Nahm and H. N. Cheng, J. Org. Chem., 57 5093 (1996).
20 H. Kwart and M. Brechbiel, J. Org. Chem., 47, 3353 (1982).
21
F. R. Benn and J. Dwyer, J. Chem. Soc., Perkin Trans. 2, 533 (1977); O. Achmatowicz and J. Szymoniak,
J. Org. Chem., 45, 4774 (1980); H. Kwart and M. Brechbiel, J. Org. Chem., 47, 3353 (1982).
22 D. A. Singleton and C. Hang, Tetrahedron Lett., 40, 8939 (1999).
23
B. B. Snider, Acc. Chem. Res., 13, 426 (1980).
24 K. Mikami and M. Shimizu, Chem. Rev., 92, 1020 (1992).
25 M. F. Salomon, S. N. Pardo, and R. G. Salomon, J. Org. Chem., 49, 2446 (1984); J. Am. Chem. Soc.,
106, 3797 (1984).
26
P. Laszlo and M. Teston-Henry, J. Phys. Org. Chem., 4 605 (1991).