Page 871 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 871
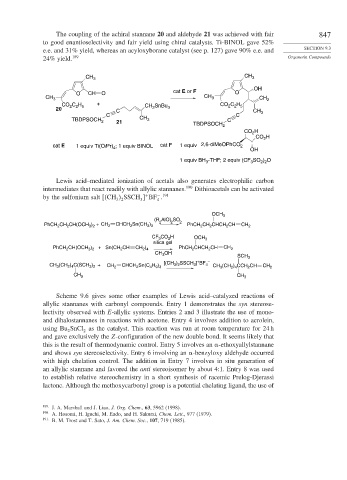
The coupling of the achiral stannane 20 and aldehyde 21 was achieved with fair 847
to good enantioselectivity and fair yield using chiral catalysts. Ti-BINOL gave 52%
e.e. and 31% yield, whereas an acyloxyborane catalyst (see p. 127) gave 90% e.e. and SECTION 9.3
24% yield. 189 Organotin Compounds
CH 3 CH 3
OH
O CH O cat E or F O
CH 3 CH 3 CH 2
CO C H + CH SnBu CO C H
2 2 5
2 2 5
20 C 2 3 CH
C C 3
TBDPSOCH 2 21 CH 3 TBDPSOCH C
2
CO H
2
CO H
2
cat E 1 equiv Ti(OiPr) ; 1 equiv BINOL cat F 1 equiv 2,6-diMeOPhCO 2 OH
4
1 equiv BH -THF; 2 equiv (CF SO ) O
3
2 2
3
Lewis acid–mediated ionization of acetals also generates electrophilic carbon
intermediates that react readily with allylic stannanes. 190 Dithioacetals can be activated
− 191
by the sulfonium salt CH SSCH BF .
+
3 2
4
3
OCH 3
(R AlO) SO
2 2 2
PhCH 2 CH 2 CH(OCH 3 ) 2 + CH 2 CHCH 2 Sn(CH 3 ) 3 PhCH 2 CH 2 CHCH 2 CH CH 2
CF 3 CO 2 H OCH 3
silica gel
PhCH 2 CH(OCH 3 ) 2 + Sn(CH 2 CH CH 2 ) 4 PhCH 2 CHCH 2 CH CH 2
CH 3 OH
SCH 3
+ –
CH 3 (CH 2 ) 4 C(SCH 3 ) 2 + CH 2 CHCH 2 Sn(C 4 H 9 ) 3 [(CH 3 ) 2 SSCH 3 ] BF 4 CH 3 (CH 2 ) 4 CCH 2 CH CH 2
CH 3 CH 3
Scheme 9.6 gives some other examples of Lewis acid–catalyzed reactions of
allylic stannanes with carbonyl compounds. Entry 1 demonstrates the syn stereose-
lectivity observed with E-allylic systems. Entries 2 and 3 illustrate the use of mono-
and dihalostannanes in reactions with acetone. Entry 4 involves addition to acrolein,
using Bu SnCl as the catalyst. This reaction was run at room temperature for 24 h
2
2
and gave exclusively the Z-configuration of the new double bond. It seems likely that
this is the result of thermodynamic control. Entry 5 involves an -ethoxyallylstannane
and shows syn stereoselectivity. Entry 6 involving an -benzyloxy aldehyde occurred
with high chelation control. The addition in Entry 7 involves in situ generation of
an allylic stannane and favored the anti stereoisomer by about 4:1. Entry 8 was used
to establish relative stereochemistry in a short synthesis of racemic Prelog-Djerassi
lactone. Although the methoxycarbonyl group is a potential chelating ligand, the use of
189
J. A. Marshall and J. Liao, J. Org. Chem., 63, 5962 (1998).
190 A. Hosomi, H. Iguchi, M. Endo, and H. Sakurai, Chem. Lett., 977 (1979).
191
B. M. Trost and T. Sato, J. Am. Chem. Soc., 107, 719 (1985).