Page 867 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 867
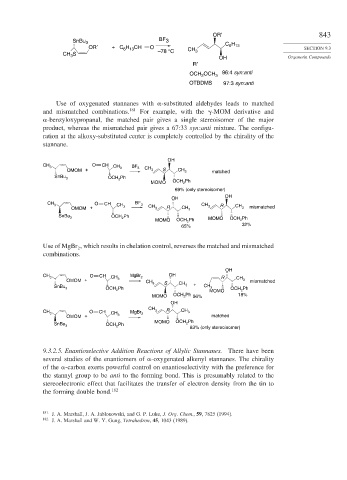
OR′ 843
SnBu 3 BF 3
OR′ + C H CH O CH C H SECTION 9.3
6 13
6 13
CH S –78 °C 3
3
OH Organotin Compounds
R′
OCH OCH 3 96:4 syn:anti
2
OTBDMS 97:3 syn:anti
Use of oxygenated stannanes with -substituted aldehydes leads to matched
and mismatched combinations. 181 For example, with the -MOM derivative and
-benzyloxypropanal, the matched pair gives a single stereoisomer of the major
product, whereas the mismatched pair gives a 67:33 syn:anti mixture. The configu-
ration at the alkoxy-substituted center is completely controlled by the chirality of the
stannane.
OH
CH O CH CH BF
3 3 3 CH
OMOM + 3 S CH 3 matched
SnBu 3 OCH Ph
2
MOMO OCH Ph
2
69% (only stereoisomer)
OH
OH
CH O CH BF
3 CH 3 3 CH R CH CH 3 R CH 3 mismatched
OMOM + 3 3
SnBu 3 OCH Ph
2
MOMO OCH Ph MOMO OCH 2 Ph
2
65% 32%
Use of MgBr , which results in chelation control, reverses the matched and mismatched
2
combinations.
OH
CH 3 O CH MgBr 2 OH R
OMOM + CH 3 CH S CH CH 3 mismatched
SnBu 3 OCH Ph 3 3 + CH MOMO OCH Ph
3
2
2
MOMO OCH Ph 56% 18%
2
OH
CH R
CH 3 O CH CH MgBr 3 CH 3
OMOM + 3 2 matched
MOMO OCH Ph
SnBu 3 OCH Ph 2 83% (only stereoisomer)
2
9.3.2.5. Enantioselective Addition Reactions of Allylic Stannanes. There have been
several studies of the enantiomers of -oxygenated alkenyl stannanes. The chirality
of the -carbon exerts powerful control on enantioselectivity with the preference for
the stannyl group to be anti to the forming bond. This is presumably related to the
stereoelectronic effect that facilitates the transfer of electron density from the tin to
the forming double bond. 182
181 J. A. Marshall, J. A. Jablonowski, and G. P. Luke, J. Org. Chem., 59, 7825 (1994).
182
J. A. Marshall and W. Y. Gung, Tetrahedron, 45, 1043 (1989).