Page 862 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 862
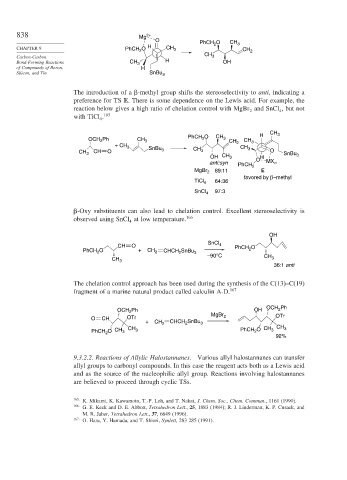
838 Mg 2+
O PhCH O CH 3
CHAPTER 9 PhCH 2 O H CH 3 2 CH 2
CH
Carbon-Carbon 3
Bond-Forming Reactions CH 3 H OH
of Compounds of Boron, H
Silicon, and Tin SnBu 3
The introduction of a -methyl group shifts the stereoselectivity to anti, indicating a
preference for TS E. There is some dependence on the Lewis acid. For example, the
reaction below gives a high ratio of chelation control with MgBr and SnCl , but not
4
2
with TiCl . 165
4
CH 3
H
OCH Ph CH 3 PhCH 2 O CH 3 CH CH 3
2
+ CH 3 SnBu 2 CH
CH 3 CH O 3 CH 3 3 O
OH CH 3 H SnBu 3
anti:syn O MX n
PhCH 2
MgBr 2 89:11 E
favored by β−methyl
TiCl 4 64:36
SnCl 4 97:3
-Oxy substituents can also lead to chelation control. Excellent stereoselectivity is
observed using SnCl at low temperature. 166
4
OH
SnCl
CH O 4 PhCH O
PhCH O + CH 2 CHCH SnBu 3 2
2
2
CH 3 –90°C CH 3
36:1 anti
The chelation control approach has been used during the synthesis of the C(13)–C(19)
fragment of a marine natural product called calculin A-D. 167
2
OCH Ph OH OCH Ph
2
O CH OTr MgBr 2 OTr
+ CH 2 CHCH 2 SnBu 3
PhCH O CH 3 CH 3 PhCH 2 O CH 3 CH 3
2
92%
9.3.2.2. Reactions of Allylic Halostannanes. Various allyl halostannanes can transfer
allyl groups to carbonyl compounds. In this case the reagent acts both as a Lewis acid
and as the source of the nucleophilic allyl group. Reactions involving halostannanes
are believed to proceed through cyclic TSs.
165 K. Mikami, K. Kawamoto, T.-P. Loh, and T. Nakai, J. Chem. Soc., Chem. Commun., 1161 (1990).
166 G. E. Keck and D. E. Abbott, Tetrahedron Lett., 25, 1883 (1984); R. J. Linderman, K. P. Cusack, and
M. R. Jaber, Tetrahedron Lett., 37, 6649 (1996).
167
O. Hara, Y. Hamada, and T. Shiori, Synlett, 283 285 (1991).