Page 856 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 856
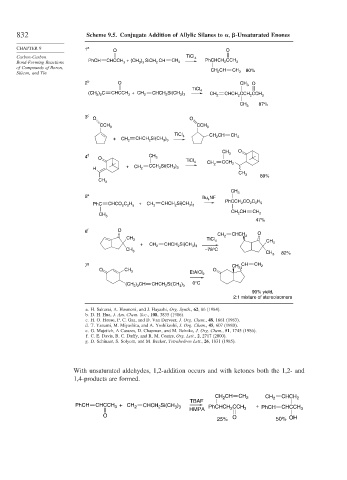
832 Scheme 9.5. Conjugate Addition of Allylic Silanes to
-Unsaturated Enones
CHAPTER 9 1 a O O
Carbon-Carbon TiCl 4 CCH
2
3
3 3
Bond-Forming Reactions PhCH CHCCH + (CH ) SiCH CH CH 2 PhCHCH 2 3
of Compounds of Boron,
CH 2 CH CH 2 80%
Silicon, and Tin
2 b O CH 3 O
TiCl
) C CHCCH + CH CHCH Si(CH ) 4
(CH 3 2 3 2 2 3 3 CH 2 CHCH CCH CCH 3
2
2
CH 87%
3
3 c O O
CCH CCH 3
3
TiCl CH CH
+ CH CHCH Si(CH ) 4 2 CH 2
2 2 3 3
CH 3 O
4 d CH
O 3
TiCl 4 CH
CCH Si(CH ) 2 CCH 2
H + CH 2 2 3 3
CH 3 89%
CH 3
CH 3
5 e NF
Bu 4 PhCCH CO C H
PhC CHCO C H 5 + CH 2 CHCH Si(CH ) 2 2 2 5
2
2
2
3 3
CH CH CH
CH 3 2 2
47%
6 f O O
CH 2 CHCH 2
CH 3 TiCl 4
+ CH 2 CHCH Si(CH ) CH 3
3 3
2
CH –78°C
3
CH 3 82%
7 g CH CH CH 2
O CH 3 O 3
EtAlCl 2
(CH ) CH CHCH Si(CH ) 0°C
2 2 2 3 3
90% yield,
2:1 mixture of stereoisomers
a. H. Sakurai, A. Hosmoni, and J. Hayashi, Org. Synth., 62, 86 (1984).
b. D. H. Hua, J. Am. Chem. Soc., 108, 3835 (1986).
c. H. O. House, P. C. Gaa, and D. Van Derveer, J. Org. Chem., 48, 1661 (1983).
d. T. Yanami, M. Miyashita, and A. Yoshikoshi, J. Org. Chem., 45, 607 (1980).
e. G. Majetich, A Casares, D. Chapman, and M. Behnke, J. Org. Chem., 51, 1745 (1986).
f. C. E. Davis, B. C. Duffy, and R. M. Coates, Org. Lett., 2, 2717 (2000).
g. D. Schinzer, S. Solyom, and M. Becker, Tetrahedron Lett., 26, 1831 (1985).
With unsaturated aldehydes, 1,2-addition occurs and with ketones both the 1,2- and
1,4-products are formed.
CH CH CH 2 CH 2 CHCH 2
2
TBAF
PhCH CHCCH + CH 2 CHCH 2 Si(CH 3 ) 3 PhCHCH CCH + PhCH
3
HMPA 2 3 CHCCH 3
O
25% O 50% OH