Page 854 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 854
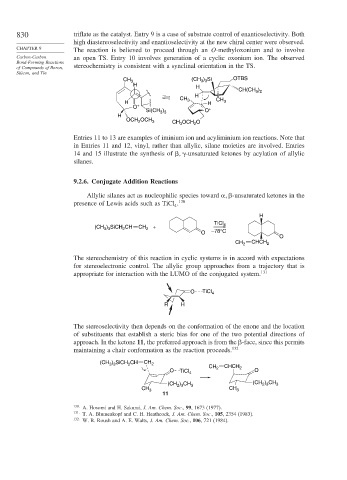
830 triflate as the catalyst. Entry 9 is a case of substrate control of enantioselectivity. Both
high diastereoselectivity and enantioselectivity at the new chiral center were observed.
CHAPTER 9 The reaction is believed to proceed through an O-methyloxonium and to involve
Carbon-Carbon an open TS. Entry 10 involves generation of a cyclic oxonium ion. The observed
Bond-Forming Reactions
of Compounds of Boron, stereochemistry is consistent with a synclinal orientation in the TS.
Silicon, and Tin
CH 3 (CH ) Si OTBS
3 3
H H
CH(CH )
3 2
H
CH CH
H 3 H 3
O +
Si(CH ) O +
3 3
H
OCH OCH 3 CH OCH O
2
3
2
Entries 11 to 13 are examples of iminium ion and acyliminium ion reactions. Note that
in Entries 11 and 12, vinyl, rather than allylic, silane moieties are involved. Entries
14 and 15 illustrate the synthesis of
-unsaturated ketones by acylation of allylic
silanes.
9.2.6. Conjugate Addition Reactions
Allylic silanes act as nucleophilic species toward
-unsaturated ketones in the
presence of Lewis acids such as TiCl . 130
4
H
TiCl
(CH ) SiCH CH CH 2 + 4
2
3 3
O –78°C
O
CH 2 CHCH 2
The stereochemistry of this reaction in cyclic systems is in accord with expectations
for stereoelectronic control. The allylic group approaches from a trajectory that is
appropriate for interaction with the LUMO of the conjugated system. 131
O TiCl 4
R H
The stereoselectivity then depends on the conformation of the enone and the location
of substituents that establish a steric bias for one of the two potential directions of
approach. In the ketone 11, the preferred approach is from the -face, since this permits
maintaining a chair conformation as the reaction proceeds. 132
(CH ) SiCH CH CH 2
2
3 3
CH CHCH
O TiCl 4 2 2 O
) CH (CH ) CH
(CH 2 3 3 2 3 3
CH 3 CH 3
11
130
A. Hosomi and H. Sakurai, J. Am. Chem. Soc., 99, 1673 (1977).
131 T. A. Blumenkopf and C. H. Heathcock, J. Am. Chem. Soc., 105, 2354 (1983).
132
W. R. Roush and A. E. Walts, J. Am. Chem. Soc., 106, 721 (1984).