Page 852 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 852
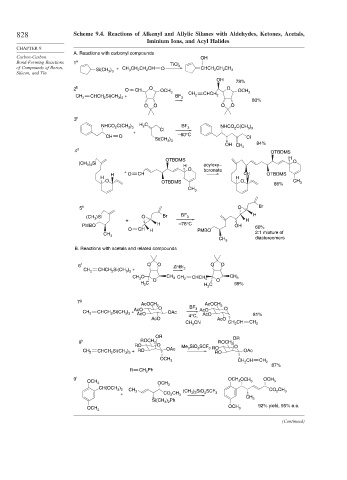
828 Scheme 9.4. Reactions of Alkenyl and Allylic Silanes with Aldehydes, Ketones, Acetals,
Iminium Ions, and Acyl Halides
CHAPTER 9
A. Reactions with carbonyl compounds
Carbon-Carbon OH
Bond-Forming Reactions 1 a
TiCl 4
of Compounds of Boron, Si(CH ) + CH CH CH CH O CHCH CH CH 3
2
3
2
2
2
Silicon, and Tin 3 3
OH 78%
2 b O CH O OCH 3 O OCH 3
CH CHCH Si(CH ) + BF CH 2 CHCH 2
2 2 3 3 3
80%
O O O O
3 c
C(CH ) H C BF
NHCO 2 3 3 2 Cl 3 NHCO C(CH )
3 3
2
+
CH O – 60°C Cl
)
Si(CH 3 3
OH CH 2 84%
4 d OTBDMS
H
OTBDMS
(CH ) Si H acyloxy– O
3 3
O boronate
+
H O CH OH OTBDMS
H H H
O OTBDMS O CH
86% 3
CH
3
5 e O Br
(CH )Si O Br BF 3 H
3
+ H
PMBO H –78°C OH 60%
O CH H PMBO
CH 2:1 mixture of
3
diastereomers
CH 3
B. Reactions with acetals and related compounds
6 f O O ZnBr O O
CHCH Si(CH ) + 2
CH 2 2 3 3
O CH
CH 3 CH 3 CH 2 CHCH 2 3
O O
H C H C 99%
3
3
7 g
AcOCH 2 AcOCH 2
AcO O BF 3 AcO O
CHCH Si(CH ) +
CH 2 2 3 3 AcO OAc 4°C, AcO 81%
AcO AcO
CH CN CH CH CH 2
2
3
OR OR
8 h ROCH 2 ROCH
RO O Me 3 SiO SCF RO 2 O
CHCH Si(CH ) + RO OAc 3 3 OAc
CH 2 2 3 3 RO
OCH
3 CH 2 CH CH 2
87%
R CH Ph
2
9 i OCH OCH OCH
OCH 3 3 3
3 OCH 3
CH(OCH )
3 2 CH 3 (CH ) SiO SCF CO CH 3
2
+ CO CH 3 3 3 3 3
2
CH
Si(CH ) Ph 3
3 2
OCH OCH 3 92% yield, 95% e.e.
3
(Continued)