Page 855 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 855
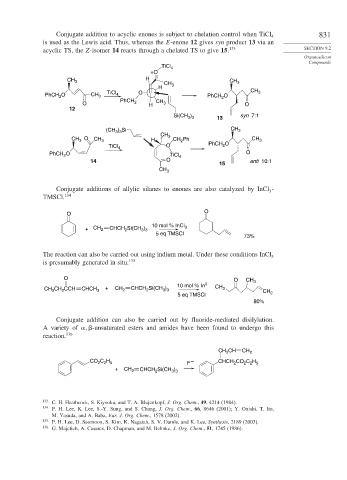
Conjugate addition to acyclic enones is subject to chelation control when TiCl 831
4
is used as the Lewis acid. Thus, whereas the E-enone 12 gives syn product 13 via an
acyclic TS, the Z-isomer 14 reacts through a chelated TS to give 15. 133 SECTION 9.2
Organosilicon
Compounds
TiCl 4
+O
CH 3 H CH 3
H CH 3 CH
PhCH O CH 3 TiCl 4 O PhCH O 3
2
2
PhCH
O 2 H CH 3 O
12
Si(CH ) 13 syn 7:1
3 3
(CH ) Si CH 3
3 3
CH 3
O CH CH Ph
CH 3 3 H 2 CH 3
2
TiCl 4 O PhCH O
PhCH O TiCl 4 O
2
14 O anti 10:1
15
CH 3
Conjugate additions of allylic silanes to enones are also catalyzed by InCl -
3
TMSCl. 134
O
O
10 mol % InCl
+ CH 2 CHCH Si(CH ) 3
3 3
2
5 eq TMSCl
73%
The reaction can also be carried out using indium metal. Under these conditions InCl 3
is presumably generated in situ. 135
O O CH 3
10 mol % In 0 CH
3 3
CH 3 CH CCH CHCH 3 + CH 2 CHCH Si(CH ) 3 CH
2
2
5 eq TMSCl 2
80%
Conjugate addition can also be carried out by fluoride-mediated disilylation.
A variety of
-unsaturated esters and amides have been found to undergo this
reaction. 136
CH CH CH 2
2
C H –
2
CO 2 2 5 CHCH CO C H
2 2 5
F
+ CH 2 CHCH Si(CH )
3 3
2
133
C. H. Heathcock, S. Kiyooka, and T. A. Blujenkopf, J. Org. Chem., 49, 4214 (1984).
134 P. H. Lee, K. Lee, S.-Y. Sung, and S. Chang, J. Org. Chem., 66, 8646 (2001); Y. Onishi, T. Ito,
M. Yasuda, and A. Baba, Eur. J. Org. Chem., 1578 (2002).
135 P. H. Lee, D. Seomoon, S. Kim, K. Nagaiah, S. V. Damle, and K. Lee, Synthesis, 2189 (2003).
136
G. Majetich, A. Casares, D. Chapman, and M. Behnke, J. Org. Chem., 51, 1745 (1986).