Page 850 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 850
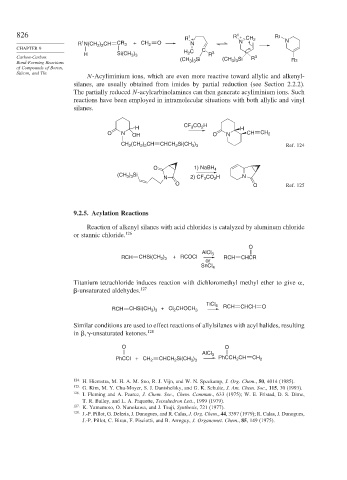
826 1 1 R1
R + R + CH 2 N
1
R N(CH ) CH CR 3 + CH 2 O N N
2 2
CHAPTER 9 C
2
H Si(CH ) H C R 3
3 3
Carbon-Carbon (CH ) Si (CH ) Si R 3
Bond-Forming Reactions 3 3 3 3 R3
of Compounds of Boron,
Silicon, and Tin
N-Acyliminium ions, which are even more reactive toward allylic and alkenyl-
silanes, are usually obtained from imides by partial reduction (see Section 2.2.2).
The partially reduced N-acylcarbinolamines can then generate acyliminium ions. Such
reactions have been employed in intramolecular situations with both allylic and vinyl
silanes.
CF CO H
H 3 2 H
O N OH O N CH CH 2
CH (CH ) CH CHCH Si(CH ) Ref. 124
2
2 2
2
3 3
O 1) NaBH 4
(CH ) Si N 2) CF CO H N
3 3
2
3
O O Ref. 125
9.2.5. Acylation Reactions
Reaction of alkenyl silanes with acid chlorides is catalyzed by aluminum chloride
or stannic chloride. 126
O
AlCl 3
) + RCOCl
RCH CHSi(CH 3 3 RCH CHCR
or
SnCl 4
Titanium tetrachloride induces reaction with dichloromethyl methyl ether to give
-unsaturated aldehydes. 127
TiCl 4
)
RCH CHSi(CH 3 3 + Cl CHOCH 3 RCH CHCH O
2
Similar conditions are used to effect reactions of allylsilanes with acyl halides, resulting
in
-unsaturated ketones. 128
O O
AlCl 3
PhCCl + CH 2 CHCH Si(CH ) PhCCH CH CH 2
2
3 3
2
124 H. Hiemstra, M. H. A. M. Sno, R. J. Vijn, and W. N. Speckamp, J. Org. Chem., 50, 4014 (1985).
125
G. Kim, M. Y. Chu-Moyer, S. J. Danishefsky, and G. K. Schulte, J. Am. Chem. Soc., 115, 30 (1993).
126
I. Fleming and A. Pearce, J. Chem. Soc., Chem. Commun., 633 (1975); W. E. Fristad, D. S. Dime,
T. R. Bailey, and L. A. Paquette, Tetrahedron Lett., 1999 (1979).
127 K. Yamamoto, O. Nunokawa, and J. Tsuji, Synthesis, 721 (1977).
128
J.-P. Pillot, G. Deleris, J. Dunogues, and R. Calas, J. Org. Chem., 44, 3397 (1979); R. Calas, J. Dunogues,
J.-P. Pillot, C. Biran, F. Pisciotti, and B. Arreguy, J. Organomet. Chem., 85, 149 (1975).