Page 846 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 846
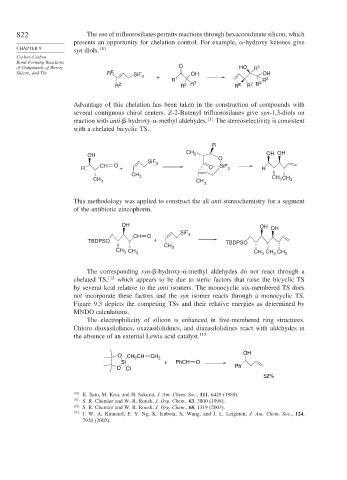
822 The use of trifluorosilanes permits reactions through hexacoordinate silicon, which
presents an opportunity for chelation control. For example, -hydroxy ketones give
CHAPTER 9 110
syn diols.
Carbon-Carbon
Bond-Forming Reactions
of Compounds of Boron, O HO R 1
Silicon, and Tin R E OH OH
SiF 3
+
R 1 3 2 R 3
R Z R 2 R R E R Z R
Advantage of this chelation has been taken in the construction of compounds with
several contiguous chiral centers. Z-2-Butenyl trifluorosilanes give syn-1,3-diols on
reaction with anti- -hydroxy- -methyl aldehydes. 111 The stereoselectivity is consistent
with a chelated bicyclic TS.
R
OH CH 3 OH OH
O
SiF 3
CH O SiF
R + O 3 R
CH 3
CH 3 CH CH 3 CH 3
3
This methodology was applied to construct the all anti stereochemistry for a segment
of the antibiotic zincophorin.
OH OH OH
SiF 3
CH O
TBDPSO + TBDPSO
CH 3
CH 3 CH 3 CH 3 CH 3 CH 3
The corresponding syn- -hydroxy- -methyl aldehydes do not react through a
chelated TS, 112 which appears to be due to steric factors that raise the bicyclic TS
by several kcal relative to the anti isomers. The monocyclic six-membered TS does
not incorporate these factors and the syn isomer reacts through a monocyclic TS.
Figure 9.3 depicts the competing TSs and their relative energies as determined by
MNDO calculations.
The electrophilicity of silicon is enhanced in five-membered ring structures.
Chloro dioxasilolanes, oxazasilolidines, and diazasilolidines react with aldehydes in
the absence of an external Lewis acid catalyst. 113
OH
O CH CH CH
2 2
Si + PhCH O
O Cl Ph
52%
110 K. Sato, M. Kira, and H. Sakurai, J. Am. Chem. Soc., 111, 6429 (1989).
111
S. R. Chemler and W. R. Roush, J. Org. Chem., 63, 3800 (1998).
112 S. R. Chemler and W. R. Roush, J. Org. Chem., 68, 1319 (2003).
113
J. W. A. Kinnaird, P. Y. Ng, K. Kubota, X. Wang, and J. L. Leighton, J. Am. Chem. Soc., 124,
7920 (2002).