Page 844 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 844
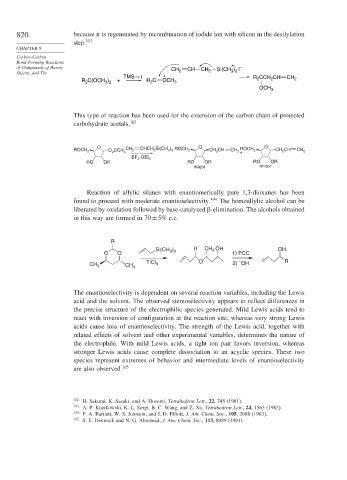
820 because it is regenerated by recombination of iodide ion with silicon in the desilylation
step. 102
CHAPTER 9
Carbon-Carbon
Bond-Forming Reactions
of Compounds of Boron, CH CH CH Si(CH ) I –
Silicon, and Tin 2 2 3 3
TMS I + R CCH CH CH
R C(OCH ) + R 2 C OCH 3 2 2 2
3 2
2
OCH 3
This type of reaction has been used for the extension of the carbon chain of protected
carbohydrate acetals. 103
O O O
ROCH 2 O CCH 3 CH 2 CHCH Si(CH ) ROCH 2 CH CH CH ROCH 2 CH CH CH 2
2
3 3
2
2 +
2
2
BF ·OEt 2
3
RO OR RO OR RO OR
major minor
Reaction of allylic silanes with enantiomerically pure 1,3-dioxanes has been
found to proceed with moderate enantioselectivity. 104 The homoallylic alcohol can be
liberated by oxidation followed by base-catalyzed -elimination. The alcohols obtained
in this way are formed in 70±5% e.e.
R
Si(CH ) R CH 3 OH OH
3 3
O O 1) PCC
TiCl O 2) OH R
–
CH 4
CH 3 3
The enantioselectivity is dependent on several reaction variables, including the Lewis
acid and the solvent. The observed stereoselectivity appears to reflect differences in
the precise structure of the electrophilic species generated. Mild Lewis acids tend to
react with inversion of configuration at the reaction site, whereas very strong Lewis
acids cause loss of enantioselectivity. The strength of the Lewis acid, together with
related effects of solvent and other experimental variables, determines the nature of
the electrophile. With mild Lewis acids, a tight ion pair favors inversion, whereas
stronger Lewis acids cause complete dissociation to an acyclic species. These two
species represent extremes of behavior and intermediate levels of enantioselectivity
are also observed. 105
102
H. Sakurai, K. Sasaki, and A. Hosomi, Tetrahedron Lett., 22, 745 (1981).
103 A. P. Kozikowski, K. L. Sorgi, B. C. Wang, and Z. Xu, Tetrahedron Lett., 24, 1563 (1983).
104 P. A. Bartlett, W. S. Johnson, and J. D. Elliott, J. Am. Chem. Soc., 105, 2088 (1983).
105
S. E. Denmark and N. G. Almstead, J. Am. Chem. Soc., 113, 8089 (1991).