Page 845 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 845
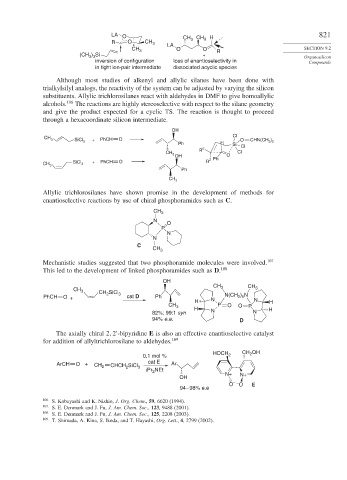
LA O CH CH H 821
R O CH 3 LA 3 3
CH 3 O O R SECTION 9.2
) Si
(CH 3 3 + Organosilicon
inversion of configuration loss of enantioselectivity in Compounds
in tight ion-pair intermediate dissociated acyclic species
Although most studies of alkenyl and allylic silanes have been done with
trialkylsilyl analogs, the reactivity of the system can be adjusted by varying the silicon
substituents. Allylic trichlorosilanes react with aldehydes in DMF to give homoallylic
alcohols. 106 The reactions are highly stereoselective with respect to the silane geometry
and give the product expected for a cyclic TS. The reaction is thought to proceed
through a hexacoordinate silicon intermediate.
OH
Cl
CH 3 SiCl + PhCH O O )
3
Ph H Si Cl CHN(CH 3 2
R E
CH Cl
3
OH O
SiCl + PhCH O R Z Ph
CH 3 3
Ph
CH
3
Allylic trichlorosilanes have shown promise in the development of methods for
enantioselective reactions by use of chiral phosphoramides such as C.
CH 3
N
O
P
N
N
C
CH 3
Mechanistic studies suggested that two phosphoramide molecules were involved. 107
This led to the development of linked phosphoramides such as D. 108
OH
CH 3 CH
CH 3 CH SiCl 3
) N
PhCH O + 2 3 cat D Ph N(CH 2 5
H N N H
CH 3 P O O P
H H
82%; 99:1 syn N N
94% e.e. D
The axially chiral 2
2 -bipyridine E is also an effective enantioselective catalyst
for addition of allyltrichlorosilane to aldehydes. 109
HOCH CH OH
0.1 mol % 2 2
cat E
ArCH O + CH 2 CHCH SiCl 3 Ar
2
i Pr NEt
2
OH N+ N+
O – – O E
94 – 98% e.e
106
S. Kobayashi and K. Nishio, J. Org. Chem., 59, 6620 (1994).
107
S. E. Denmark and J. Fu, J. Am. Chem. Soc., 123, 9488 (2001).
108 S. E. Denmark and J. Fu, J. Am. Chem. Soc., 125, 2208 (2003).
109
T. Shimada, A. Kina, S. Ikeda, and T. Hayashi, Org. Lett., 4, 2799 (2002).