Page 843 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 843
The stereochemistry is consistent with approach of the silane anti to the methyl 819
substituent.
SECTION 9.2
H
PhCH 2
PhCH 2 H Organosilicon
CH 3 O Compounds
O Cl Sn CH 3
Cl Sn 4 O
4
O
H H
In contrast, BF showed very low stereoselectivity, consistent with its inability to form
3
a chelate.
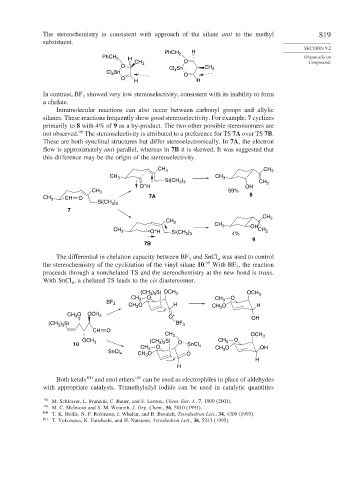
Intramolecular reactions can also occur between carbonyl groups and allylic
silanes. These reactions frequently show good stereoselectivity. For example, 7 cyclizes
primarily to 8 with 4% of 9 as a by-product. The two other possible stereoisomers are
98
not observed. The stereoselectivity is attributed to a preference for TS 7A over TS 7B.
These are both synclinal structures but differ stereoelectronically. In 7A, the electron
flow is approximately anti parallel, whereas in 7B it is skewed. It was suggested that
this difference may be the origin of the stereoselectivity.
CH 3 CH 3
CH 3 CH 3
Si(CH ) CH
3 3
+
O H OH 2
59%
CH 3
CH 3 CH O 7A 8
Si(CH )
3 3
7
CH 3
CH 3 CH
CH 3 O H Si(CH ) 3 4% OH CH 2
+
3 3
9
7B
The differential in chelation capacity between BF and SnCl was used to control
3 4
the stereochemistry of the cyclization of the vinyl silane 10. 99 With BF , the reaction
3
proceeds through a nonchelated TS and the stereochemistry at the new bond is trans.
With SnCl , a chelated TS leads to the cis diastereomer.
4
(CH ) Si OCH 3 OCH 3
3 3
O O
CH 3 CH 3
BF 3 CH O H CH O H
3
3
CH O OCH 3 O + OH
3
(CH ) Si BF 3
3 3
CH O
CH 3 OCH 3
OCH (CH ) Si CH 3 O
10 3 3 3 O SnCl 4
CH 3 O CH O OH
3
SnCl 4 CH O O
3
H
H
Both ketals 100 and enol ethers 101 can be used as electrophiles in place of aldehydes
with appropriate catalysts. Trimethylsilyl iodide can be used in catalytic quantities
98
M. Schlosser, L. Franzini, C. Bauer, and F. Leroux, Chem. Eur. J., 7, 1909 (2001).
99
M. C. McIntosh and S. M. Weinreb, J. Org. Chem., 56, 5010 (1991).
100 T. K. Hollis, N. P. Robinson, J. Whelan, and B. Bosnich, Tetrahedron Lett., 34, 4309 (1993).
101
T. Yokozawa, K. Furuhashi, and H. Natsume, Tetrahedron Lett., 36, 5243 (1995).