Page 838 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 838
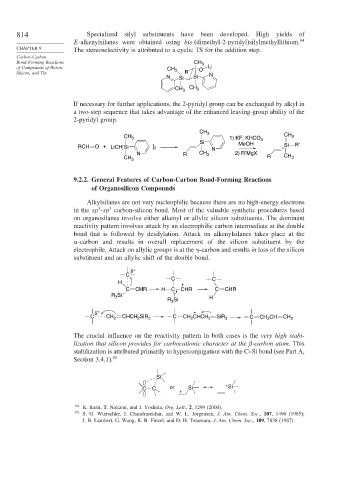
814 Specialized silyl substituents have been developed. High yields of
E-alkenylsilanes were obtained using bis-(dimethyl-2-pyridyl)silylmethyllithium. 84
CHAPTER 9 The stereoselectivity is attributed to a cyclic TS for the addition step.
Carbon-Carbon
Bond-Forming Reactions CH 3
of Compounds of Boron, CH 3 O Li
Silicon, and Tin R N
N Si Si
CH 3 CH 3
If necessary for further applications, the 2-pyridyl group can be exchanged by alkyl in
a two-step sequence that takes advantage of the enhanced leaving-group ability of the
2-pyridyl group.
CH 3
CH 3 1) KF, KHCO 3 CH 3
Si MeOH R′
RCH O + LiCH[Si ] 2 N Si
N R CH 3 2) R′MgX
CH 3 R CH 3
9.2.2. General Features of Carbon-Carbon Bond-Forming Reactions
of Organosilicon Compounds
Alkylsilanes are not very nucleophilic because there are no high-energy electrons
3
3
in the sp -sp carbon-silicon bond. Most of the valuable synthetic procedures based
on organosilanes involve either alkenyl or allylic silicon substituents. The dominant
reactivity pattern involves attack by an electrophilic carbon intermediate at the double
bond that is followed by desilylation. Attack on alkenylsilanes takes place at the
-carbon and results in overall replacement of the silicon substituent by the
electrophile. Attack on allylic groups is at the -carbon and results in loss of the silicon
substituent and an allylic shift of the double bond.
δ +
C
C C
H
+
C CHR H C CHR C CHR
R Si
3
R Si H
3
δ + +
C CH 2 CHCH SiR 3 C CH CHCH 2 SiR 3 C CH CH CH 2
2
2
2
The crucial influence on the reactivity pattern in both cases is the very high stabi-
lization that silicon provides for carbocationic character at the ß-carbon atom. This
stabilization is attributed primarily to hyperconjugation with the C–Si bond (see Part A,
Section 3.4.1). 85
Si
+
C C or Si Si
+
84 K. Itami, T. Nokami, and J. Yoshida, Org. Lett., 2, 1299 (2000).
85
S. G. Wierschke, J. Chandrasekhar, and W. L. Jorgensen, J. Am. Chem. Soc., 107, 1496 (1985);
J. B. Lambert, G. Wang, R. B. Finzel, and D. H. Teramura, J. Am. Chem. Soc., 109, 7838 (1987).